ΧβΡΩΡΎ»ί
ΓΨΧβΡΩΓΩΡ≥Μ·―ß–Υ»Λ–ΓΉιάϊ”Οœ¬ΆΦΉΑ÷ΟΫχ––““Υα““θΞΚœ≥…ΚΆΖ÷άκΒΡ Β―ιΧΫΨΩΘ§«κΜΊ¥π“‘œ¬Έ Χβ

Θ®1Θ©–¥≥ωΚœ≥…““Υα““θΞΒΡΜ·―ßΖΫ≥Χ Ϋ_______________________________ΓΘ
Θ®2Θ©“«ΤςbΒΡΟϊ≥Τ________Θ§ΆΦ÷–ΤπάδΡΐΜΊΝςΉς”ΟΒΡ «______Θ®ΧνΘαΓΔΘβΓΔΘψΓΔΘδΓΔeΘ©ΓΘ
Θ®3Θ©ΈΣΝΥΧαΗΏ““Υα““θΞΒΡ≤ζ¬ Ω…≤…»ΓΒΡ¥κ © _______________________________
Θ®4Θ©Ψ≠Ιΐ0.5hΦ”»»Ζ¥”ΠΚσΘ§ΫΪΖ¥”ΠΉΑ÷Οc÷–¥÷≤ζΤΖΉΣ“Τ÷Νd÷–Ϋχ––’τΝσΓΘ
Έο÷ | 98.3%≈®ΝρΥα | ““Υα““θΞ | ““Υα | ““¥Φ | ““Ο― | Υ° |
Ζ–Βψ | 338ΓφΘ§ | 77.1Γφ | 118Γφ | 78.5Γφ | 34.6Γφ | 100Γφ |
ΗυΨί…œ±μΖ÷ΈωΘ§’τΝσΚσΒΟΒΫΒΡ““Υα““θΞ÷–Θ§Ήν”–Ω…ΡήΚ§”–________________‘”÷ ΓΘ
ΓΨ¥πΑΗΓΩ CH3COOHΘΪCH3CH2OH![]() CH3COOCH2CH3ΘΪH2O άδΡΐΙή Θα ΫΪCH3COOCH2CH3ΦΑ ±’τΝσΖ÷άκΘΜΖ¥”ΠΈ¬Ε»≤Μ“ΥΙΐΗΏΘ§Φθ…ΌCH3COOH ΓΔCH3CH2OHΒΡΜ”ΖΔΘΜΖ¥”ΠΈοΩΊ÷ΤΈόΥ°ΧθΦΰΘ§≈®ΝρΥαΒΡΈϋΥ°Ής”Ο”–άϊ”ΎΤΫΚβœρ’ΐΖ¥”ΠΖΫœρ“ΤΕ·Θ§ΧαΗΏ≤ζ¬ ΓΘΘ®–¥≥ωΤδ÷–“ΜΧθΦ¥Ω…Θ© CH3CH2OH
CH3COOCH2CH3ΘΪH2O άδΡΐΙή Θα ΫΪCH3COOCH2CH3ΦΑ ±’τΝσΖ÷άκΘΜΖ¥”ΠΈ¬Ε»≤Μ“ΥΙΐΗΏΘ§Φθ…ΌCH3COOH ΓΔCH3CH2OHΒΡΜ”ΖΔΘΜΖ¥”ΠΈοΩΊ÷ΤΈόΥ°ΧθΦΰΘ§≈®ΝρΥαΒΡΈϋΥ°Ής”Ο”–άϊ”ΎΤΫΚβœρ’ΐΖ¥”ΠΖΫœρ“ΤΕ·Θ§ΧαΗΏ≤ζ¬ ΓΘΘ®–¥≥ωΤδ÷–“ΜΧθΦ¥Ω…Θ© CH3CH2OH
ΓΨΫβΈωΓΩΘ®1Θ©Κœ≥…““Υα““θΞΒΡΜ·―ßΖΫ≥Χ ΫΈΣCH3COOHΘΪCH3CH2OH![]() CH3COOCH2CH3ΘΪH2OΘΜΘ®2Θ©“«ΤςbΒΡΟϊ≥ΤάδΡΐΙήΘ§a «÷±–ΈάδΡΐΙήΘ§ΆΦ÷–ΤπάδΡΐΜΊΝςΉς”ΟΒΡ «aΘΜΘ®3Θ©”…”ΎθΞΜ·Ζ¥”Π «Ω…ΡφΖ¥”ΠΘ§ΧαΗΏ““Υα““θΞΒΡ≤ζ¬ Ω…≤…»ΓΒΡ¥κ ©ΈΣΘΚΫΪCH3COOCH2CH3ΦΑ ±’τΝσΖ÷άκΘΜΖ¥”ΠΈ¬Ε»≤Μ“ΥΙΐΗΏΘ§Φθ…ΌCH3COOH CH3CH2OHΒΡΜ”ΖΔΘΜΖ¥”ΠΈοΩΊ÷ΤΈόΥ°ΧθΦΰΘ§≈®ΝρΥαΒΡΈϋΥ°Ής”Ο”–άϊ”ΎΤΫΚβœρ’ΐΖ¥”ΠΖΫœρ“ΤΕ·Θ§ΧαΗΏ≤ζ¬ ΘΜΘ®4Θ©”…±μΗώ÷–ΒΡ ΐΨίΩ…÷ΣΘ§““¥Φ”κ““Υα““θΞΒΡΖ–ΒψΫ”ΫϋΘ§‘ρ’τΝσΚσΒΟΒΫΒΡ““Υα““θΞ÷–Θ§Ήν”–Ω…ΡήΚ§”–CH3CH2OH ‘”÷ ΓΘ
CH3COOCH2CH3ΘΪH2OΘΜΘ®2Θ©“«ΤςbΒΡΟϊ≥ΤάδΡΐΙήΘ§a «÷±–ΈάδΡΐΙήΘ§ΆΦ÷–ΤπάδΡΐΜΊΝςΉς”ΟΒΡ «aΘΜΘ®3Θ©”…”ΎθΞΜ·Ζ¥”Π «Ω…ΡφΖ¥”ΠΘ§ΧαΗΏ““Υα““θΞΒΡ≤ζ¬ Ω…≤…»ΓΒΡ¥κ ©ΈΣΘΚΫΪCH3COOCH2CH3ΦΑ ±’τΝσΖ÷άκΘΜΖ¥”ΠΈ¬Ε»≤Μ“ΥΙΐΗΏΘ§Φθ…ΌCH3COOH CH3CH2OHΒΡΜ”ΖΔΘΜΖ¥”ΠΈοΩΊ÷ΤΈόΥ°ΧθΦΰΘ§≈®ΝρΥαΒΡΈϋΥ°Ής”Ο”–άϊ”ΎΤΫΚβœρ’ΐΖ¥”ΠΖΫœρ“ΤΕ·Θ§ΧαΗΏ≤ζ¬ ΘΜΘ®4Θ©”…±μΗώ÷–ΒΡ ΐΨίΩ…÷ΣΘ§““¥Φ”κ““Υα““θΞΒΡΖ–ΒψΫ”ΫϋΘ§‘ρ’τΝσΚσΒΟΒΫΒΡ““Υα““θΞ÷–Θ§Ήν”–Ω…ΡήΚ§”–CH3CH2OH ‘”÷ ΓΘ

ΓΨΧβΡΩΓΩ―«œθθΘ¬»(ClNO) «”–ΜζΚœ≥…÷–ΒΡ÷Ί“Σ ‘ΦΝΘ§ΙΛ“Β…œΩ…”ΟNO”κCl2Κœ≥…Θ§ΜΊ¥πœ¬Ν–Έ ΧβΘΚ
(1)ΓΣΕ®ΧθΦΰœ¬Θ§ΒΣ―θΜ·Έο”κ–ϋΗΓ‘Ύ¥σΤχ÷–ΒΡΚΘ―ΈΝΘΉ”œύΜΞΉς”Ο ±Μα…ζ≥…―«œθθΘ¬»Θ§…φΦΑ»»Μ·―ßΖΫ≥Χ ΫΚΆΤΫΚβ≥Θ ΐ»γœ¬±μΘΚ
–ρΚ≈ | »»Μ·―ßΖΫ≥Χ Ϋ | ΤΫΚβ≥Θ ΐ |
ΔΌ | 2NO2(g) +NaCl(s) | K1 |
ΔΎ | 4NO2(g) +2NaCl(s) | k2 |
Δέ | 2NO(g)+Cl2(g) | K3 |
K3=_______(”ΟK1ΓΔK2±μ Ψ)ΓΘ
(2)25Γφ ±Θ§œρΧεΜΐΈΣ2L«“¥χΤχ―ΙΦΤΒΡΚψ»ίΟή±’»ίΤς÷–Ά®»κ0.08molNOΚΆ0.04molCl2ΖΔ…ζΖ¥”ΠΘΚ2NO(g)+Cl2(g) ![]() 2ClNO(g) ΓςH3ΓΘ
2ClNO(g) ΓςH3ΓΘ
ΔΌ œ¬Ν–Οη ωΡήΥΒΟςΗΟΖ¥”Π“―¥οΒΫΤΫΚβΉ¥Χ§ΒΡ «_____Θ®Χν–ρΚ≈Θ©
a.v’ΐ(Cl2)=2vΡφ(NO) b.»ίΤςΡΎΜλΚœΤχΧεΒΡΟήΕ»±Θ≥÷≤Μ±δ
c.»ίΤςΡΎΤχΧε―Ι«Ω±Θ≥÷≤Μ±δ d.»ίΤςΡΎΜλΚœΤχΧεΒΡΤΫΨυœύΕ‘Ζ÷Ή”÷ ΝΩ±Θ≥÷≤Μ±δ
ΔΎ»τΖ¥”ΠΤπ ΦΚΆΤΫΚβ ±Έ¬Ε»œύΆ§Θ§≤βΒΟΖ¥”ΠΙΐ≥Χ÷–―Ι«ΩΘ®P)Υφ ±ΦδΘ®t)ΒΡ±δΜ·»γΆΦI«ζœΏaΥυ ΨΘ§‘ρΓςH3______0(ΧνΓΑ >Γ±ΓΔΓΑ < Γ±ΜρΓΑ≤Μ»ΖΕ®"Θ©ΘΜ»τΤδΥϊΧθΦΰœύΆ§Θ§ΫωΗΡ±δΡ≥“ΜΧθΦΰ ±Θ§≤βΒΟΤδ―Ι«Ω(P)Υφ ±ΦδΘ®t)ΒΡ±δΜ·»γΆΦI«ζœΏbΥυ ΨΘ§³tΗΡ±δΒΡΧθΦΰ «_________ΓΘ
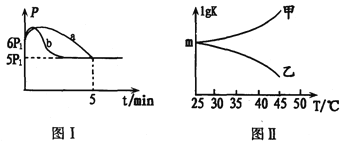
ΔέΆΦII «ΦΉΓΔ““Ά§―ßΟηΜφ…œ ωΖ¥”ΠΤΫΚβ≥Θ ΐΒΡΕ‘ ΐ÷ΒΘ®lgK)”κΈ¬Ε»ΒΡ±δΜ·ΙΊœΒΘ§Τδ÷–’ΐ»ΖΒΡ«ζœΏ «____(ΧνΓΑΦΉΓ±ΜρΓΑ““Γ±Θ©ΘΜm÷ΒΈΣ_______ΓΘ


