��Ŀ����
14�������йص������Һ���������ʵ���Ũ�ȹ�ϵ��ȷ���ǣ�����������O��1mol/LNaCl��Һ�У�c��Na+��=c��Cl-����c��OH-��=c��H+��
����0.1mol/LNa2CO3��Һ�У�c��OH-��=c��HCO3-��+2c��H2C03��+c��H+��
����0.1mol/LNaHCO3��Һ�м�������O��1mol/LNaOH��Һ��c��CO32-����c��Na+����c��OH-����c��H+��
�ܳ����£�CH3COONa��CH3COOH�����ҺPH=7��c��Na+����c��CH3COO-��
| A�� | �٢� | B�� | �ۢ� | C�� | �٢� | D�� | �ڢ� |
���� ��NaCl��Һ�����ԣ���c��Na+��=c��Cl-����c��OH-��=c��H+����
�ڸ���̼������Һ�е������غ��жϣ�
�۷�Ӧ������Ϊ̼���ƣ�����̼������Ӳ���ˮ�⣬��c��Na+����2c��CO32-����
�ܻ����ҺpH=7����c��OH-��=c��H+������ϵ���غ��жϣ�
��� �⣺��O��1mol/LNaCl��Һ�����ԣ�����Һ������Ũ�ȴ�СΪ��c��Na+��=c��Cl-����c��OH-��=c��H+�����ʢ���ȷ��
����0.1mol/LNa2CO3��Һ�����������غ㣺c��OH-��=c��HCO3-��+2c��H2C03��+c��H+�����ʢ���ȷ��
����0.1mol/LNaHCO3��Һ�м�������O��1mol/LNaOH��Һ����Ӧ������Ϊ̼���ƣ�����̼������Ӳ���ˮ�⣬��c��Na+����2c��CO32-������Һ����ȷ������Ũ�ȴ�СΪ��c��Na+����c��CO32-����c��OH-����c��H+�����ʢ۴���
�ܳ����£�CH3COONa��CH3COOH�����ҺpH=7����c��OH-��=c��H+�������ݵ���غ��֪��c��Na+��=c��CH3COO-�����ʢܴ���
��ѡA��
���� ���⿼��������Ũ�ȴ�С�Ƚϣ���Ŀ�Ѷ��еȣ���ȷ��Ӧ���������Ϊ���ؼ���ע�������ε�ˮ��ԭ��������غ㡢�����غ㼰�����غ�ĺ��壬�ܹ������ε�ˮ��ԭ�����غ�֪ʶ�жϸ�����Ũ�ȴ�С��

| ʱ�� | c��A��/��mol•L-1�� | c��B��/��mol•L-1�� | c��C��/��mol•L-1�� |
| 0min | 1 | 3 | 0 |
| ��2min | 0.8 | 2.6 | 0.4 |
| ��4min | 0.4 | 1.8 | 1.2 |
| ��6min | 0.4 | 1.8 | 1.2 |
��1��p=2��a=1��b=2��ȡ��С������
��2����2min����4min�ڣ�A��ƽ����Ӧ����v��A��=0.2 mol•L-1•min-1
��3�����ӿ�ʼ����4min����ƽ��ʱ����Ӧ�ų�������Ϊ234kJ����÷�Ӧ�ġ�H=-195��
| A�� | ������Ʊ�ʵ���У���NaNO3��KCl�Ļ��Һ���Ȳ�Ũ�����о������������ȹ���ʱ�����Է��������� | |
| B�� | �ؽᾧʱ����Һ��ȴ�ٶ�Խ���õ��ľ������Խ�� | |
| C�� | ��ѹ���˲���ʱ��Ӧ����ֽ���벼��©���ڣ���ֽ��СӦ�Դ���©���ھ����ܽ�ȫ��С��ס | |
| D�� | ����֧�Թ��зֱ��1ml��ˮ�Ҵ���1.5g���ӹ��壬�ټӵ������ƣ��Ƚ��Ҵ��������ǻ�����ԭ�ӵĻ����� |
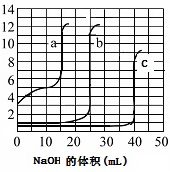
| A�� | ��ͼ��֪����cΪNaOH�ζ����� | |
| B�� | ��ͼ��֪����aΪNaOH�ζ����� | |
| C�� | �ζ�ʵ�������ɫʯ����Һ��ָʾ�� | |
| D�� | ��ͼ��֪����bΪNaOH�ζ����� |
| ѹǿ/Mpa �������/% �·�/�� | 1.0 | 2.0 | 3.0 |
| 810 | 54.0 | a | b |
| 915 | c | 75.0 | d |
| 1000 | e | f | 83.0 |
| A�� | �÷�Ӧ�ġ�S��0 | B�� | 915�棬2.0 MPaʱE��ת����Ϊ40% | ||
| C�� | b��f | D�� | K��1000�棩��K��810�棩 |
| A�� | ��λʱ��������amolX��ͬʱ����4amolZ | |
| B�� | �����ڵ��������ٱ仯 | |
| C�� | Z������������Y���������ʱ�ֵΪ1��2 | |
| D�� | ������������ܶȲ������仯 |
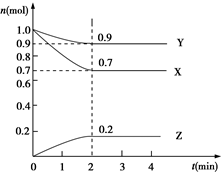 ij�¶�ʱ����2L���ܱ������У�X��Y��Z�������ʣ�����XΪ���壬Y��ZΪ���壩�����ʵ�����ʱ��ı仯������ͼ��ʾ��
ij�¶�ʱ����2L���ܱ������У�X��Y��Z�������ʣ�����XΪ���壬Y��ZΪ���壩�����ʵ�����ʱ��ı仯������ͼ��ʾ����1����ͼ���������ݽ��з������÷�Ӧ�Ļ�ѧ����ʽΪX+3Y?2Z��
��2����Ӧ�ӿ�ʼ��2����ĩ����Z��Ũ�ȱ仯��ʾ��ƽ����Ӧ����Ϊv��Z��=0.05mol•��L•min��-1��
��3���������������䣬ֻ�ı�һ����Ӧ����������Ӧ���ʵı仯������пո��
������ĸ��A������ B����С C�����䣩
| �ı����� | �����¶� | ����X���� | ʹ�ô��� | ����������� |
| ���ʱ仯 |