��Ŀ����
4��ʵ��������0.1mol•L-1��BaCl2��Һ�ɷ������ν��У���һ�Σ���������ƽ��ȡ5.2g��ˮBaCl2���壮�ڶ��Σ��ܽ�����0.1mol•L-1��BaCl2��Һ����һ�β��������¼�����A�������벦��0.2g����B�������벦����0������C������ƽ���ߵ������ϸ���һ�Ÿɾ��ĵ���������ֽ��������ƽ���ߵ���ĸʹ��ƽƽ�⣻D��ȡ��ҩƷ��������Ż�������ڣ�E���������������Ӿ�������ƽƽ�⣻F���������Ϸ���5g���룮
��1������ȷ�IJ���˳���ǣ�����ţ���B��C��F��A��E��D��B
��2����E�����У�ֻȱ��������ʱ���������������������ʹ�������������ձ��У�
��3���ڶ��β�����Ӧ�Ƚ�5.2g BaCl2����������ˮ�ܽ⣬�ܽ������ʹ�õ���Ҫ�������ձ�����������Ȼ����Һת������ƿ�У��پ�ϴ�ӡ����ݡ�ҡ�Ⱥɵõ�0.1mol•L-1BaCl2��Һ��
��4�����в���ʹ���Ƶ�BaCl2��ҺŨ��ƫ�͵���AC��
A����������������ϣ�BaCl2���������Ͻ��г���
B��ѡ�õ�����ƿ������������ˮ
C������ҡ�Ⱥ�Һ���½����ּ�ˮ���̶���
D������ʱ����Һ��
��5��100mL����ƿ��ʢ��100mL 0.101mol•L-1��BaCl2��Һ����������ϡ�ͳ�Ũ��Ϊ0.100mol•L-1��BaCl2��Һ����ѡ�õ������У�10mL��Ͳ��1mL��Һ�ܣ���ȷ��ȡ0.10��1.00mL��Һ������ʽ�ζ��ܡ���ͷ�ιܣ���IJ�����������1mL��Һ���Ƴ�0.99mL��0.1010mol•L-1��BaCl2��Һ��Ȼ���ý�ͷ�ι���ʣ�µ�99.01mL 0.1010mol•L-1��BaCl2��Һ�м�ˮ���̶��ߣ��������Ƶ�0.100mol•L-1��BaCl2��Һ����
���� ��1����ƽ����ǰӦ�ŵ�ˮƽ�����ϣ������벦����̶��ߣ�����������ĸʹ����ƽ�⣻��ƽʹ��ʱ���������룬����������ʹ��ƽ�ٴδﵽƽ��ʱ�����Ե�������ֱ������ƽ�⣻��¼���������Ҫ����ʵ�����ģ������ӽ�����Ż�����У�
��2����������ҩƷʱ�����Ҫ����������ҩƷ��Ӧ��������ʹ�������������ձ��У�
��3����������һ�����ʵ���Ũ��Һ����ѡ���������
��4�������������������ʵ����ʵ�������Һ�������Ӱ�죬����c=$\frac{n}{V}$������������
��5����������100mL0.100mol•L-1��BaCl2��Һ�������0.1010mol•L-1��BaCl2��Һ�����ΪVmL��Ȼ�������Һϡ�Ͷ���CŨVŨ=CϡVϡ��������������Һ�������Ȼ������Һ���Ƴ�һ�������0.1010mol•L-1��BaCl2��Һ��Ȼ���ý�ͷ�ι���ʣ�µ�0.1010mol•L-1��BaCl2��Һ�м�ˮ���̶��ߣ��������Ƶ�0.100mol•L-1��BaCl2��Һ��
��� �⣺��1����ƽ����ȷʹ�ò��裺a����ƽ�ŵ�ˮƽ̨�ϣ������Ƶ������˵���̶ȣ�
b������ƽ��ƽ����ĸʹָ��ָ���ֶ��̵����룬������ƫת�ĸ�����ͬ��c���������ƽ�����̣����������ƽ�����̣������������ڱ���ϵ�λ�ã�ֱ�������ָ�ƽ�⣮Ҫ�����Ӽ�ȡ���룬Ҫ������ţ�Ҫ�Ӵ�С��
d���������=���������+�����Ӧ�Ŀ̶ȣ�
e�������ģ�
������ȷ�IJ���˳��Ϊ��BCFAEDB��
�ʴ�Ϊ��BCFAEDB��
��2����������ҩƷʱ������ҩƷֻȱ��������ʱ��Ӧ��������ʹ�������������ձ��У�
�ʴ�Ϊ����������ʹ�������������ձ��У�
��3������һ�����ʵ���Ũ����Һ�IJ������裺���㡢�������ܽ⡢��Һ��ϴ�ӡ����ݡ�ҡ�ȣ��ܽ�ʱ������Ӧ�����ձ��У��ò��������Ͻ��裻
�ʴ�Ϊ���ձ���������������ƿ��ϴ�ӣ����ݣ�ҡ�ȣ�
��4��A����������������ϣ�BaCl2���������Ͻ��г��������³�ȡ������ƫС�����ʵ����ʵ���ƫС����Һ��Ũ��ƫ�ͣ���Aѡ��
B��ѡ�õ�����ƿ������������ˮ�������ʵ����ʵ�������Һ��������������Ӱ�죬��Һ��Ũ�Ȳ���Ӱ�죬��B��ѡ��
C������ҡ�Ⱥ�Һ���½����ּ�ˮ���̶��ߣ�������Һ�����ƫ����Һ��Ũ��ƫ�ͣ���Cѡ��
D������ʱ����Һ�浼����Һ���ƫС����ҺŨ��ƫ�ߣ���D��ѡ��
��ѡ��AC��
��5����������100mL0.100mol•L-1��BaCl2��Һ�������0.1010mol•L-1��BaCl2��Һ�����ΪVmL��������Һϡ�Ͷ���CŨVŨ=CϡVϡ��֪��
0.1010mol•L-1��VmL=100mL��0.100mol•L-1
���V=99.01mL������1mL��Һ���Ƴ�0.99mL��0.1010mol•L-1��BaCl2��Һ��Ȼ���ý�ͷ�ι���ʣ�µ�99.01mL 0.1010mol•L-1��BaCl2��Һ�м�ˮ���̶��ߣ��������Ƶ�0.100mol•L-1��BaCl2��Һ��
�ʴ�Ϊ����1mL��Һ���Ƴ�0.99mL��0.1010mol•L-1��BaCl2��Һ��Ȼ���ý�ͷ�ι���ʣ�µ�99.01mL 0.1010mol•L-1��BaCl2��Һ�м�ˮ���̶��ߣ��������Ƶ�0.100mol•L-1��BaCl2��Һ��
���� ���⿼����һ�����ʵ���Ũ����Һ�����ƣ���Ϥ��ƽ��ʹ�÷�������ȷ����ԭ���Ͳ��������ǽ���ؼ���ע���������ķ����ͼ��ɣ�

| A�� | ��Ȼ��--CO | B�� | �ƾ�--C2H5OH | C�� | ���ʯ--Si | D�� | ʯ��--CaCO3 |
K Na KCl NaCl?
�۵㣨�棩 63.6 97.8 770 801?
�е㣨�棩 774 882.9 1500 1413?
����ƽ���ƶ�ԭ��������֪��Na��KCl��Ӧ��ȡ�����ص������¶���?��������
| A�� | ����770�� | B�� | 850�� | C�� | ����882.9�� | D�� | 1413��1500�� |
| A�� | ���ӻ�ԭ��Y2-��Z-��������c��b | B�� | �⻯����ȶ���H2Y��HZ | ||
| C�� | ԭ�Ӱ뾶X��W����һ������X��W | D�� | �縺��Z��Y��W��Z |
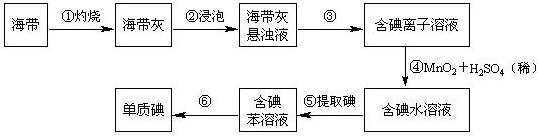

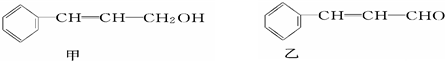
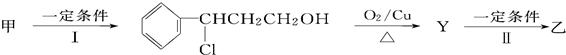
 ��ע����Ӧ��������
��ע����Ӧ��������