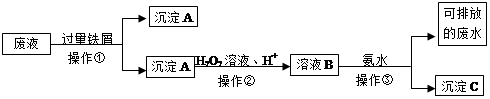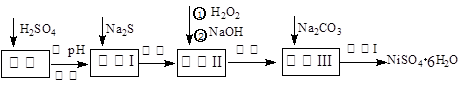��Ŀ����
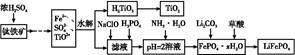
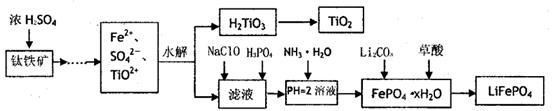
ij���������̿�MnO2Լ70����Al2O3������п��ZnSԼ80����FeS������ͬ����MnO2��Zn���ɵ��ԭ�ϣ���
��֪����A��MnSO4��ZnSO4��Fe2��SO4��3��Al2��SO4��3�Ļ��Һ��
��IV�еĵ�ⷴӦʽΪMnSO4+ZnSO4+2H2O  MnO2+Zn+2H2SO4��
MnO2+Zn+2H2SO4��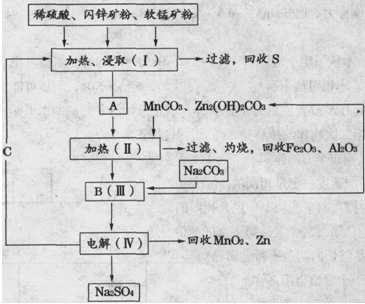
��1��A�����ڻ�ԭ������� ��
��2��MnCO3��Zn2��OH��2CO3�������� ������Ҫ���ȵ�ԭ���� ��C�Ļ�ѧʽ�� ��
��3�����з��������ӷ���ʽΪ �� ��
��4����������������е���ģ�����ʯ�⣬�蹺��Ļ���ԭ���� ��
��1��MnSO4 ��2�֣�
��2��������Һ��pH��ʹFe3+��Al3+�����ɳ�����2�֣����ٽ�Fe3+��Al3+��ˮ�⣨2�֣��� H2SO4��2�֣�
��3��Mn2+ + CO32- = MnCO3����2�֣� 2Zn2+ + 2CO32- + H2O = Zn2��OH��2CO3 ��+ CO2����2�֣�
��4����������ᣨ2�֣�
���������������1���Ƚ���Ϣ��A�����̿���Ԫ�ػ��ϼ۵ı仯��֪��MnԪ�ػ��ϼ���+4�۽���Ϊ+2�ۣ�����A�л�ԭ����ΪMnSO4��
��2���ɹ������̿�֪��MnCO3��Zn2��OH��2CO3�����þ��ǵ���pH��ʹFe3+��Al3+������ȫ��Fe3+��Al3+���������γɽ��壬���������������������������������м��ȵ�Ŀ���Ǽ��ٳ������ɣ���ֹ������֣���ʹ�γɽ����������������������Ҳ���ɳ�������������ȡ���ȡ��Ҫ���ᣬ�ɢڿ�֪CΪ���ᣬѭ�����á�
��3����ҺB�к���Mn2+��Zn2+����������ͼ������Na2CO3������MnCO3�� Zn2��OH��2CO3�����ӷ���ʽΪ��Mn2+ + CO32- = MnCO3���� 2Zn2+ + 2CO32- + H2O = Zn2��OH��2CO3 ��+ CO2��
��4��������ͼ��֪�������̼���ơ����ᣬ���Գ���ʯ�⣬�蹺��Ļ���ԭ���Ǵ�������ᡣ
���㣺���⿼�黯ѧ���̵ķ������Լ������á����ӷ���ʽ����д��������ԭ��Ӧ��

 ��ѧ��������������Ͼ���ѧ������ϵ�д�
��ѧ��������������Ͼ���ѧ������ϵ�д� �ϴ�̸�������������νӽ̳��Ͼ���ѧ������ϵ�д�
�ϴ�̸�������������νӽ̳��Ͼ���ѧ������ϵ�д�Ϊ̽��ij����ҩX����ɣ���������ʵ�飺
�������ϣ�
�ٿ���ҩX���ܵ���ɿ��Ա�ʾΪ��MgmAln(OH)p(CO3)q(SiO3)r��m��n��p��q��rΪ��0����������
��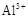 ��pH=5.0ʱ������ȫ��
��pH=5.0ʱ������ȫ��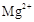 ��pH=8.8ʱ��ʼ������pH=11.4ʱ������ȫ��
��pH=8.8ʱ��ʼ������pH=11.4ʱ������ȫ��
ʵ����̣�
| ���� | ʵ����� | ʵ������ |
| I | ��X�ķ�ĩ�м���������� | ��������A���õ���ɫ��Һ |
| II | ������õ���Һ�еμӰ�ˮ������pH��5~ 6������ | ���ɰ�ɫ����B |
| III | �����B�мӹ���NaOH��Һ | ����ȫ���ܽ� |
| IV | ��II�õ�����Һ�еμ�NaOH��Һ������pH��12 | ���ɰ�ɫ����C |
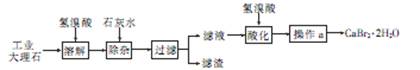
��1����������A��ʹ����ʯ��ˮ����ǣ�A�Ļ�ѧʽ�� ��
��2��II������B��Ӧ�����ӷ���ʽ�� ��
��3��III��B�ܽⷴӦ�����ӷ���ʽ�� ��
��4������C�Ļ�ѧʽ�� ��
��5��������n(A)�Un(B)�Un(C)=1�U2�U3����X�Ļ�ѧʽ�� ��
��ҵ������п����Ҫ�ɷ�ΪZnS��������Fe2O3�����ʣ�Ϊԭ������ZnSO4��7H2O�Ĺ����������£�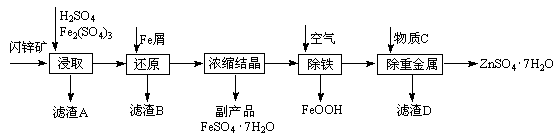
��1��������A�пɻ��һ�ֵ���ɫ�ǽ������ʵĸ���Ʒ���仯ѧʽΪ ��
��2����ȡ������Fe2(SO4)3�������� ����ȡʱFe2(SO4)3��ZnS������Ӧ�Ļ�ѧ����ʽΪ ��
��3���������̿�����Һ��pH��5.4���ң��÷�Ӧ�����ӷ���ʽΪ ���ù����ڿ�����ڴ������һ��������ԡ��ͷ��װ�ã���Ŀ���� ��
��4���û������ؽ���������������CΪ ��
��5������п���ܽ�����¶�֮��Ĺ�ϵ���±���
| �¶�/�� | 0 | 20 | 40 | 60 | 80 | 100 |
| �ܽ��/g | 41.8 | 54.1 | 70.4 | 74.8 | 67.2 | 60.5 |
�ӳ��ؽ����������п��Һ�л������п�����ʵ�����Ϊ �� �����ˡ����