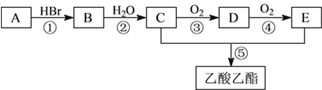
��Ŀ����
����Ŀ����ͼ��һ���绯ѧ���̵�ʾ��ͼ����ش��������⣺
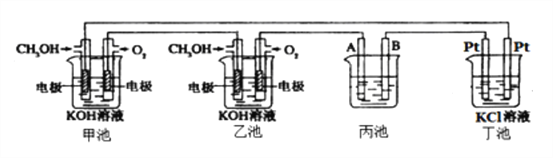
��1��ͨ��CH3OH�ĵ缫�ĵ缫��ӦʽΪ__________________��
��2��������ͭ�ľ����أ����һ��ʱ����ձ��е���ҺŨ�Ȼ�______����������С������������A�缫��Ӧʽ_______________________����֪��ͭ�к���Zn��Ag���ʣ���
��3�������е��з�̪��ʵ�鿪ʼ��۲쵽������_____________________�����ز�����Ĥ���,�������������ܻ���KOH��Һ�Ӵ����õ�KClO��H2������Ӧ�Ļ�ѧ��Ӧ����ʽΪ____________________________________________________��
��4�����ס��������������ͨ������Ϊ20L����״�������ҷ�Ӧ��ȫ����������ͨ�����صĵ���Ϊ__________�������ڳ���F=9.65��l04Cmol-1������������ܲ�������������Ϊ______L����״������
���𰸡� CH3OH-6e-+8OH-=CO32-+6H2O ��С Zn-2e-=Zn2+ Cu-2e-=Cu2+ ���缫��������ð�����ҵ缫������Һ��� KCl��H2O![]() KClO��H2�� 3.44��l05C 80
KClO��H2�� 3.44��l05C 80
����������1���״���CԪ�صĻ��ϼ����ߣ���ͨ��CH3OH�ĵ缫Ϊ������ʧȥ���ӷ���������Ӧ���缫��ӦΪCH3OH-6e-+8OH-=CO32-+6H2O����2������A��������������AΪ��������ͭ������ͭΪ��������AΪ��ͭ��п��ͭʧ���Ӳ���п���Ӻ�ͭ���ӣ���ͭ�����������õ��Ӳ���ͭ���ʣ��ʵ��һ��ʱ����ձ��е���ҺŨ�Ȼ��С��A�缫��ӦʽΪZn-2e-=Zn2+��Cu2++2e-=Cu����3������Ȼ�����Һ�������������������������أ���Һ�ʼ��ԣ��ɹ۲쵽���缫��������ð�����ҵ缫������Һ��죬����ܷ�ӦʽΪ2KCl+2H2O![]() 2KOH+H2��+Cl2������4���ס����е�������Ӧ��ΪO2+4e-+2H2O=4OH-����������ͨ�����صĵ���Ϊ
2KOH+H2��+Cl2������4���ס����е�������Ӧ��ΪO2+4e-+2H2O=4OH-����������ͨ�����صĵ���Ϊ![]() ��4��9.65��l04C/mol=3.44��l05C���ɵ����غ��֪O2��4e-��2H2����2Cl2��������������Ϊ���������4�������Զ�������ܲ�������������Ϊ20L��4=80L��
��4��9.65��l04C/mol=3.44��l05C���ɵ����غ��֪O2��4e-��2H2����2Cl2��������������Ϊ���������4�������Զ�������ܲ�������������Ϊ20L��4=80L��

 ��������һ���þ�ϵ�д�
��������һ���þ�ϵ�д� Сѧ��10����Ӧ����ϵ�д�
Сѧ��10����Ӧ����ϵ�д�