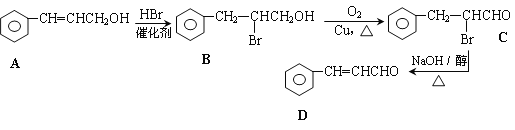
��Ŀ����
����Ŀ������˵����ȷ���� ( )
A. CH4(g)+2O2(g)��CO2(g)+2H2O(g)��H=-801.3kJmol-1 ���ۣ�CH4��ȼ����Ϊ801��3kJ/mol
B. ϡ��Һ����H+(aq)+OH-(aq)=H2O(l)��H=-57.3kJ/mol ���ۣ��������백ˮ��ϡ��Һ��Ϻ�������1mol H2O�����ų�57��3kJ������
C. Sn(s����)![]() Sn(s����)��H��+2.1kJ/mol (����Ϊ��ĩ״) ���ۣ�����Ʒ�ں���Ķ�������ת��Ϊ��������
Sn(s����)��H��+2.1kJ/mol (����Ϊ��ĩ״) ���ۣ�����Ʒ�ں���Ķ�������ת��Ϊ��������
D. C(s��ʯī)+O2(g)=CO2(g) ��C(s�����ʯ)+O2(g)=CO2(g) ��H����395kJ/mol ���ۣ���ͬ�����½��ʯ���ʱ�ʯī�ȶ�
���𰸡�C
��������
A��ȼ�����������ȶ��IJ���(Һ̬ˮ)�ų�����������������̬ˮ����A����B����ˮ��������ʣ��������������������Էų�������С��57.3 kJ������ϡ�����백ˮ��Ӧ���к��ȴ���-57.3 kJ/mol����B����C����Sn(s����)![]() Sn(s����)��֪���¶ȵ���13.2��Cʱ������ת��Ϊ�����������Է�ĩ״���ڣ���C��ȷ��D���ɢ�C(s��ʯī)+O2(g)=CO2(g)��H1=-393.5kJmol-1����C(s�����ʯ)+O2(g)=CO2(g)��H2=-393.0kJmol-1�����-�ڵã�C(S��ʯī)=C(S�����ʯ)��H=+1.9kJmol-1�����ʯ��������ʯī�������������ʵ�������Խ��Խ���ȶ�����ʯī�Ƚ��ʯ�ȶ�����D����ѡC��
Sn(s����)��֪���¶ȵ���13.2��Cʱ������ת��Ϊ�����������Է�ĩ״���ڣ���C��ȷ��D���ɢ�C(s��ʯī)+O2(g)=CO2(g)��H1=-393.5kJmol-1����C(s�����ʯ)+O2(g)=CO2(g)��H2=-393.0kJmol-1�����-�ڵã�C(S��ʯī)=C(S�����ʯ)��H=+1.9kJmol-1�����ʯ��������ʯī�������������ʵ�������Խ��Խ���ȶ�����ʯī�Ƚ��ʯ�ȶ�����D����ѡC��