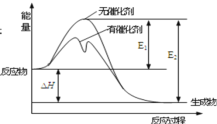
ΧβΡΩΡΎ»ί
ΓΨΧβΡΩΓΩ(1)‘Ύ“ΜΕ®ΧθΦΰœ¬N2”κH2Ζ¥”Π…ζ≥…NH3Θ§“―÷Σ≤πΩΣ1 mol HΓΣHΦϋΓΔ1 mol NΓΣHΦϋΓΔ1 mol NΓ‘NΦϋΖ÷±π–η“ΣΒΡΡήΝΩ «436 kJΓΔ391 kJΓΔ945.7 kJΘ§‘ρN2”κH2Ζ¥”Π…ζ≥…NH3ΒΡ»»Μ·―ßΖΫ≥Χ ΫΈΣΘΚ_____ΓΘ
(2)N2H4ΚΆH2O2ΜλΚœΩ…ΉςΜπΦΐΆΤΫχΦΝΘ§“―÷ΣΘΚ16 g“ΚΧ§N2H4ΚΆΉψΝΩ―θΤχΖ¥”Π…ζ≥…N2(g)ΚΆH2O(l)Θ§Ζ≈≥ω310.6 kJ ΒΡ»»ΝΩΘ§Ζ¥”ΠN2H4(l)+O2(g)=N2(g)+2H2O(l)ΒΡΓςHΘΫ_____kJmol-1ΓΘ
“―÷ΣΘΚ2H2O2(l)=O2(g)+2H2O(l) ΓςHΘΫ-196.4 kJmol-1Θ°N2H4ΚΆH2O2Ζ¥”Π…ζ≥…N2(g)ΚΆH2O(l)ΒΡ»»Μ·―ßΖΫ≥Χ ΫΈΣ_____ΓΘ
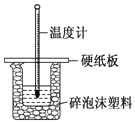
(3) Β―ι “”Ο50 mL 0.50 molL-1―ΈΥα”κ50 mLΡ≥≈®Ε»ΒΡNaOH»ή“Κ‘Ύ»γœ¬ΆΦΥυ ΨΉΑ÷Ο÷–Ζ¥”ΠΘ§Ά®Ιΐ≤βΕ®Ζ¥”ΠΙΐ≥Χ÷–ΥυΖ≈≥ωΒΡ»»ΝΩΩ…ΦΤΥψ÷–ΚΆ»»ΓΘΗΟΉΑ÷Ο”–“Μ¥ΠΟςœ‘ΒΡ¥μΈσΘ§ΗΟ¥Π¥μΈσ «»±…Ό“Μ÷÷≤ΘΝß“«ΤςΘ§ΗΟ“«ΤςΒΡΟϊ≥ΤΈΣ_____ΘΜ Β―ι “ΧαΙ©ΝΥ0.50 molL-1ΚΆ0.55 molL-1ΝΫ÷÷≈®Ε»ΒΡNaOH»ή“ΚΘ§”Π―Γ‘ώ_____ molL-1ΒΡ»ή“ΚΫχ–– Β―ιΓΘ
ΓΨ¥πΑΗΓΩN2(g)+3H2(g)=2NH3(g)ΓςH=-92.3kJmol-1 -621.2 kJmol-1 N2H4(l)+2H2O2(l)=N2(g)+4H2O(l)ΓςH=-817.6kJmol-1ΘΜ ΜΖ–Έ≤ΘΝßΫΝΑηΑτ 0.55
ΓΨΫβΈωΓΩ
Θ®1Θ©‘ΎΖ¥”ΠN2+3H22NH3÷–Θ§ΕœΝ―3molH-HΦϋΘ§1mol N»ΐNΦϋ“ΣΈϋ ’ΡήΝΩΘ§–Έ≥…6mol N-HΦϋΘ§“Σ ΆΖ≈ΡήΝΩΘ§ΓςH=Ψ…ΦϋΕœΝ―Έϋ ’ΒΡΡήΝΩΓΣ–¬Φϋ–Έ≥… ΆΖ≈ΒΡΡήΝΩΘ§¥ζ»κ ΐΨίΦ¥Ω…ΦΤΥψΘΜ
Θ®2Θ©16g“ΚΧ§N2H4ΚΆΉψΝΩ―θΤχΖ¥”Π…ζ≥…N2(g)ΚΆH2O(l)Θ§Ζ≈≥ω310.6kJΒΡ»»ΝΩΘ§»»Μ·―ßΖΫ≥Χ ΫΈΣN2H4(l)+O2(g)=N2(g)+2H2O(l)ΓςH=-621.2 kJmol-1ΔΌΘ§Ι ¥πΑΗΈΣ-621.2ΘΜ2H2O2(l)=2H2O(l)+O2(g)ΓςH=-196.4kJmol-1ΔΎΗυΨίΗ«ΥΙΕ®¬…Θ§ΔΌ+ΔΎΩ…ΒΟΒΫ¥πΑΗΘΜ
Θ®3Θ©ΗυΨίΝΩ»»ΦΤΒΡΙΙ‘λΩ…÷ΣΗΟΉΑ÷ΟΒΡ»±…Ό“«Τς «ΜΖ–Έ≤ΘΝßΫΝΑηΑτΘΜ Β―ι “ΧαΙ©ΝΥ0.50molL-1ΚΆ0.55molL-1ΝΫ÷÷≈®Ε»ΒΡNaOH»ή“ΚΘ§ΈΣΝΥ ΙΖ¥”Π≥δΖ÷Θ§NaOH”ΠΙΐΝΩΘ§Υυ“‘―Γ‘ώ0.55molL-1ΒΡ»ή“ΚΫχ–– Β―ιΓΘ
Θ®1Θ©‘ΎΖ¥”ΠN2+3H22NH3÷–Θ§ΕœΝ―3molH-HΦϋΘ§1mol N»ΐNΦϋΙ≤Έϋ ’ΒΡΡήΝΩΈΣΘΚ3ΓΝ436kJ+945.7kJ=2253.7kJΘ§…ζ≥…2mol NH3Θ§Ι≤–Έ≥…6mol N-HΦϋΘ§Ζ≈≥ωΒΡΡήΝΩΈΣΘΚ6ΓΝ391kJ=2346kJΘ§Έϋ ’ΒΡ»»ΝΩ…ΌΘ§Ζ≈≥ωΒΡ»»ΝΩΕύΘ§ΗΟΖ¥”ΠΈΣΖ≈»»Ζ¥”ΠΘ§Ζ≈≥ωΒΡ»»ΝΩΈΣΘΚ2346kJ-2253.7kJ=92kJΘΜΙ ¥πΑΗΈΣN2(g)+3H2(g)=2NH3(g)ΓςH=-92.3kJmol-1ΘΜ
Θ®2Θ©16g“ΚΧ§N2H4ΚΆΉψΝΩ―θΤχΖ¥”Π…ζ≥…N2(g)ΚΆH2O(l)Θ§Ζ≈≥ω310.6kJΒΡ»»ΝΩΘ§»»Μ·―ßΖΫ≥Χ ΫΈΣN2H4(l)+O2(g)=N2(g)+2H2O(l)ΓςH=-621.2 kJmol-1ΔΌΘ§Ι ¥πΑΗΈΣ-621.2ΘΜ
2H2O2(l)=2H2O(l)+O2(g)ΓςH=-196.4kJmol-1ΔΎΗυΨίΗ«ΥΙΕ®¬…Θ§ΔΌ+ΔΎΒΟΘΚN2H4(l)+2H2O2(l)=N2(g)+4H2O(l)ΓςH=-817.6kJmol-1Θ§Ι ¥πΑΗΈΣN2H4(l)+2H2O2(l)=N2(g)+4H2O(l)ΓςH=-817.6kJmol-1ΘΜ
Θ®3Θ©ΗυΨίΝΩ»»ΦΤΒΡΙΙ‘λΩ…÷ΣΗΟΉΑ÷ΟΒΡ»±…Ό“«Τς «ΜΖ–Έ≤ΘΝßΫΝΑηΑτΘΜ Β―ι “ΧαΙ©ΝΥ0.50molL-1ΚΆ0.55molL-1ΝΫ÷÷≈®Ε»ΒΡNaOH»ή“ΚΘ§ΈΣΝΥ ΙΖ¥”Π≥δΖ÷Θ§NaOH”ΠΙΐΝΩΘ§Υυ“‘―Γ‘ώ0.55molL-1ΒΡ»ή“ΚΫχ–– Β―ιΘ§Ι ¥πΑΗΈΣΜΖ–Έ≤ΘΝßΫΝΑηΑτΘΜ0.55ΓΘ

ΓΨΧβΡΩΓΩΙΊ”Ύ‘ΣΥΊΗθ![]() ΒΡ–≈œΔ»γœ¬ΘΚ
ΒΡ–≈œΔ»γœ¬ΘΚ
Δώ![]()
»ή“Κ÷–¥φ‘Ύ–Έ Ϋ |
|
|
|
|
―’…Ϊ | άΕΉœ…Ϊ | ¬Χ…Ϊ | ≥»Κλ…Ϊ | ΜΤ…Ϊ |
Δρ![]() ΈΣΡ―»ή”ΎΥ°ΒΡΜ“άΕ…ΪΙΧΧε
ΈΣΡ―»ή”ΎΥ°ΒΡΜ“άΕ…ΪΙΧΧε
Δσ![]() ΚΆ
ΚΆ![]() ‘Ύ»ή“Κ÷–Ω…œύΜΞΉΣΜ·ΘΚ
‘Ύ»ή“Κ÷–Ω…œύΜΞΉΣΜ·ΘΚ![]()
“Έ¬œ¬Θ§≥θ Φ≈®Ε»ΈΣ![]() ΒΡ
ΒΡ![]() »ή“Κ÷–
»ή“Κ÷–![]() Υφ
Υφ![]() ΒΡ±δΜ·»γΆΦΥυ ΨΘ§“‘œ¬ΥΒΖ®’ΐ»ΖΒΡ «
ΒΡ±δΜ·»γΆΦΥυ ΨΘ§“‘œ¬ΥΒΖ®’ΐ»ΖΒΡ «

A.»τ“―÷Σ![]() ”κ
”κ![]() ΒΡ―ß–‘÷ œύΥΤΘ§‘Ύ
ΒΡ―ß–‘÷ œύΥΤΘ§‘Ύ![]() »ή“Κ÷–÷πΒΈΦ”»κNaOH»ή“Κ÷±÷ΝΙΐΝΩΘ§Ω…Ιέ≤λΒΫ≤ζ…ζΜ“άΕ…Ϊ≥ΝΒμΘ§»ΜΚσ≥ΝΒμ÷πΫΞ»ήΫβ–Έ≥…Έό…Ϊ»ή“Κ
»ή“Κ÷–÷πΒΈΦ”»κNaOH»ή“Κ÷±÷ΝΙΐΝΩΘ§Ω…Ιέ≤λΒΫ≤ζ…ζΜ“άΕ…Ϊ≥ΝΒμΘ§»ΜΚσ≥ΝΒμ÷πΫΞ»ήΫβ–Έ≥…Έό…Ϊ»ή“Κ
B.”…ΆΦΩ…÷ΣΘ§»ή“ΚΥα–‘‘ω¥σΘ§![]() ΒΡΤΫΚβΉΣΜ·¬ Φθ–Γ
ΒΡΤΫΚβΉΣΜ·¬ Φθ–Γ
C.ΗυΨίAΒψ ΐΨίΘ§Ω…ΦΤΥψ≥ωΗΟΉΣΜ·Ζ¥”ΠΒΡΤΫΚβ≥Θ ΐΈΣ![]()
D.BΒψ»ή“Κ÷–Θ§3c![]()
![]()