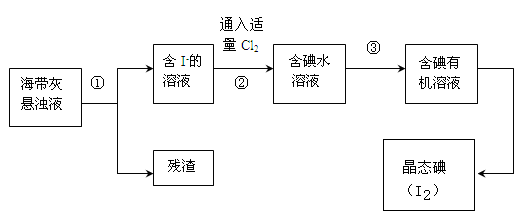
��Ŀ����
������ʾ��þ�뱥��̼��������Һ��Ӧ������������Ͱ�ɫ�����ijͬѧ���������ʵ�鷽������֤���̽����Ӧԭ����
(1)�������
ʵ�����ɰֽ��ȥþ����������Ĥ���������ʢ���������з�̪�ı���̼��������Һ���Թ��У�Ѹ�ٷ�Ӧ�������������ݺͰ�ɫ�������Һ��dz���졣
��ͬѧ�Է�Ӧ�в����İ�ɫ�������������²²⣺
�²�1����ɫ���������Ϊ________________��
�²�2����ɫ���������ΪMgCO3��
�²�3����ɫ����������Ǽ�ʽ̼��þ[xMgCO3��yMg(OH)2]��
(2)��ƶ���ʵ��ȷ�����ﲢ��֤�²⣺
| ʵ����� | ʵ�� | ʵ������ | ���� |
| ʵ��� | ��ʵ������ռ����������ȼ | �ܰ���ȼ�ա���������ɫ���� | ������ɷ�Ϊ________ |
| ʵ��� | ��ȡʵ����еİ�ɫ�����ϴ�ӣ���������________ | ��________________ __________________ __________________ | ��ɫ��������ܺ���MgCO3 |
| ʵ��� | ȡʵ����еij���Һ�������м�������CaCl2ϡ��Һ | ������ɫ���� | ����Һ�д���________ |
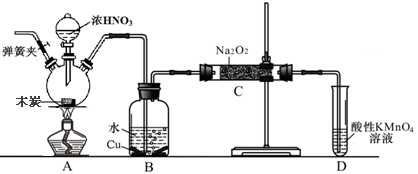
(3)Ϊ��һ��ȷ��ʵ���IJ����ƶ���ʵ�鷽������ͼ��ʾ��

��ȡʵ��������ø�������İ�ɫ������22.6 g����ּ��������ٲ�������Ϊֹ����ʹ�ֽ����������ȫ������װ��A��B�С�ʵ���װ��A����1.8 g��װ��B����8.8 g����ȷ����ɫ������Ļ�ѧʽ��__________________________ ______________________________________________��
(4)���ϻ�ѧ����ͻ�ѧƽ���ƶ�ԭ������Mg�ͱ���NaHCO3��Һ��Ӧ�����������ݺͰ�ɫ�������ԭ��____________________________________��
��(1)Mg(OH)2
(2)����������ϡ����(��������)���۲������ݣ�����ȫ���ܽ⡡��CO32��
(3)2MgCO3��Mg(OH)2��Mg(OH)2��2MgCO3��Mg3(OH)2(CO3)2
(4)NaHCO3��Һ�д���ƽ�⣺HCO32��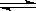 H����CO32����H2O
H����CO32����H2O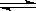 H����OH����Mg��H����Ӧ����H2��Mg2����Mg2����OH����CO32����Ӧ����������Mg(OH)2��2MgCO3����H����OH����CO32����Ũ�Ⱦ���С����ʹ������ƽ��������ƶ�
H����OH����Mg��H����Ӧ����H2��Mg2����Mg2����OH����CO32����Ӧ����������Mg(OH)2��2MgCO3����H����OH����CO32����Ũ�Ⱦ���С����ʹ������ƽ��������ƶ�
����

��������һ����Ҫ���л�����ԭ�ϣ��Ʊ��������ԭ����95%�Ҵ���80%���ᣨ������ˮϡ��Ũ���ᣩ����ϸ���廯�Ʒ�ĩ�ͼ������Ƭ���÷�Ӧ��ԭ�����£�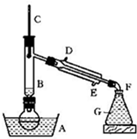
NaBr + H2SO4 �� NaHSO4 + HBr
CH3CH2OH + HBr CH3CH2Br + H2O
CH3CH2Br + H2O
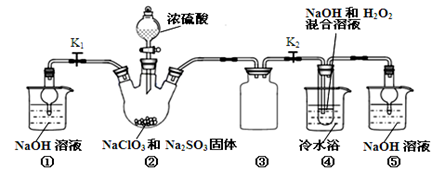
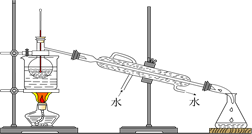
ij����С������ʵ�����Ʊ��������װ������ͼ���������±���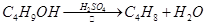
| ���� ���� | �Ҵ� | ������ | 1��2-�������� | ���� | Ũ���� |
| �ܶ�/g��cm-3 | 0.79 | 1.46 | 2.2 | 0.71 | 1.84 |
| �۵㣨�棩 | ��130 | ��119 | 9 | ��116 | 10 |
| �е㣨�棩 | 78.5 | 38.4 | 132 | 34.6 | 338 |
| ��ˮ�е��ܽ�ȣ�g/100gˮ�� | ���� | 0.914 | 1 | 7.5 | ���� |
��ش��������⡣
��1������ҩƷ֮ǰ�����IJ�����:_________________��ʵ����е�;��������δ�������Ƭ���䴦���ķ�����__________________��
��2��װ��B�������dz���ʹ���������������һ��Ŀ����_____________���¶ȼƵ��¶�Ӧ������_____________֮�䡣
��3����Ӧʱ�п�������SO2��һ�ֺ���ɫ���壬��ѡ������������Һ��ȥ�����壬�йص����ӷ���ʽ��___________��______________���˲�������___________����д�����������ƣ��н��У�ͬʱ���з��롣
��4��ʵ���в���80%���ᣬ��������98%Ũ���ᣬһ������Ϊ�˼��ٸ���Ӧ����һ������Ϊ��_______________________��
��5���ֲ�Ʒ�к��е���Ҫ�л�Һ��������_____________��Ϊ��һ���Ƶô����������飬�Դֲ�Ʒ����ˮϴ�ӡ���Һ���ټ�����ˮCaCl2������______________������
SiO2��SO2��CO2����������������ǵĻ�ѧ���ʾ���һ���������ԣ�Mg��Na�Ļ�ѧ����Ҳ����һ�������ԡ�
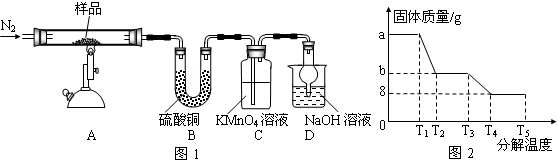
ij��ȤС������ͼ��ʾװ�ý���Mg��SO2��Ӧ��ʵ�顣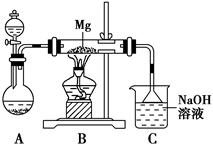
(1)ѡ����ȡSO2�ĺ����Լ�________(����)��
��ŨHCl����ŨH2SO4����Na2SO3���塡��CaSO3����
(2)����װ�û����Ż����Ż��ķ�����________________________________________��װ��C��NaOH��Һ��������___________________________________________________________
(3)��ͬѧ�Ʋ�Mg��SO2�ķ�Ӧ��Mg��CO2�ķ�Ӧ���ƣ���÷�Ӧ����ʽΪ_________________________________________��
��ͬѧ���Ʋ��ǣ�2Mg��3SO2 2MgSO3��S����ͬѧ���Ʋ��ǣ�3Mg��SO2
2MgSO3��S����ͬѧ���Ʋ��ǣ�3Mg��SO2 2MgO��MgS��Ҫ��֤�ס��ҡ�����λͬѧ���Ʋ��Ƿ���ȷ����ͬѧ������ʵ��̽����
2MgO��MgS��Ҫ��֤�ס��ҡ�����λͬѧ���Ʋ��Ƿ���ȷ����ͬѧ������ʵ��̽����
��֪��MgSO3��MgS������ˮ���������ᷢ�����ֽⷴӦ�ų����壻H2S����ͨ��CuSO4��Һ�г��ֺ�ɫ������
��ѡ�Լ���2 mol��L��1���ᡢ2 mol��L��1���ᡢ����ˮ��2 mol��L��1 NaOH��Һ��Ʒ����Һ������ʯ��ˮ��2 mol��L��1 CuSO4��Һ����������Ʒ��ѡ��
| ��� | ʵ�鲽�� | Ԥ������ͽ��� |
| �� | ȡ������Ӧ�����ù������Թ��� | |
| �� | ���Թ��еĹ��������μ�____________���Թܿ����ϴ����ܵĵ���������������ͨ��ʢ��________���Թ��� | ���Թ��е�________�����ͬѧ�Ʋ���ȷ�����Թ��еĹ���δ��ȫ�ܽ⣬��________������ͬѧ�Ʋ���ȷ |
��������ʵ��̽������֤����ͬѧ�Ʋ���ȷ�IJ�����Ԥ��������
_____________________________________________________________��
(4)����ʵ����Ҫ100 mL 2 mol��L��1�����ᣬ����ʱѡ��________(ѡ��10 mL��25 mL��50 mL��100 mL)��Ͳ��ȡ36.5%�ܶ�Ϊ1.19 g��mL��1��Ũ��������Ϊ________mL��