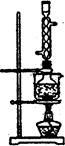
��Ŀ����
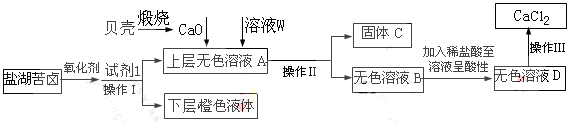
���������纣���������к��зḻ�ĵ�Ԫ�أ���Ҫ�Ե⻯����ʽ���ڡ���һ��ѧ����С���ú���Ϊԭ����ȡ�����ⵥ�ʣ����ǽ��������ճɻң���ˮ����һ��ʱ��(���õ⻯�����ܽ���ˮ��)���õ�����������Һ��Ȼ������ʵ��������ȡ���ʵ⣺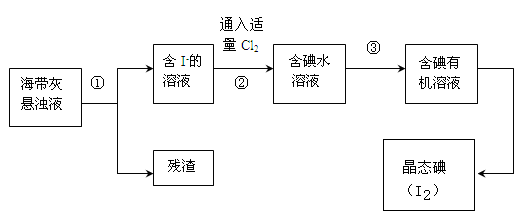
��1��ָ����ȡ��Ĺ������йص�ʵ��������ƣ���_________����________��
��2�����̢��г�������Cl2��Ӧ�����ӷ���ʽΪ_____________________��
��3�������������õ��л��Լ�������___________(ֻ��һ��)������ѡ��������__________________��
��4���Ӻ�����л���Һ����ȡ��ͻ����л���Һ������Ҫ�������۲���ͼ��ʾʵ��װ�ã�ָ�������֮�������Ը���_______________________________________________________��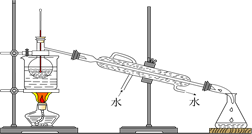
(5)���������������ʱ��ʹ��ˮԡ��ԭ����______________________��
��10�֣�(1) �ٹ��ˣ�����ȡ��(2) Cl2 + 2I-��2Cl- + I2��
(3) CCl4��CCl4��ˮ�����ܣ�����CCl4�ܽ�ȴ�����ˮ�е��ܽ�ȡ�
(4) ��ʯ�������¶ȼ�ˮ����λ�ô�������ˮˮ���������
(5)�����ڿ����¶Ȳ�ʹ���Ⱦ��ȡ�
���������������1������Һ�Ͳ����Թ������ķ����ǹ��ˡ����ʵ��������л��ܼ��У���˴ӵ�ˮ�л�ȡ�ⵥ�ʲ�����ȡ�ķ�����
��2��������������ǿ�ڵ��ʵ⣬�����͵����ӷ�Ӧ���ɵⵥ�ʣ���Ӧ�����ӷ���ʽΪCl2 + 2I-��2Cl- + I2��
��3����ȡ����ѡȡ���ǣ���������ȡ���е��ܽ�ȴ�����ԭ�ܼ��е��ܽ�ȣ����ʺ���ȡ������Ӧ����ȡ����ԭ���ܼ����ܻ��ܡ�CCl4��ˮ�����ܣ�����CCl4�ܽ�ȴ�����ˮ�е��ܽ�ȣ����Կ��������Ȼ�̼����ȡ����
��4����������У������������ƿ���Ľ�ˮ�ڣ��¶Ƚϸߵ�����������ˮ�¶ȼ��罵�ͣ�������ը�������ܣ�Ϊ��ֹ������ը������������������ƿ���Ŀ��dz�ˮ�ڣ�����ȴˮ����Ӧ�����½��ϳ�������ʱ�¶ȼƲ������������¶ȶ�������Һ�¶ȣ�����¶ȼ�ˮ����Ӧ�÷���������ƿ֧�ܳ��ڴ�������������ƿ����ֱ�Ӽ��ȣ���Ҫ��ʯ������
��5���þƾ���ֱ�Ӽ����¶ȱ仯�죬��ˮԡ����������ƿ����Һ���ȱȽϾ��ȣ����Բ���ˮԡ���ȵ�ԭ���������ڿ����¶Ȳ�ʹ���Ⱦ��ȡ�
���㣺������ȡ�Լ�����ʵ��������й��жϺ�Ӧ��

������ʾ��þ�뱥��̼��������Һ��Ӧ������������Ͱ�ɫ�����ijͬѧ���������ʵ�鷽������֤���̽����Ӧԭ����
(1)�������
ʵ�����ɰֽ��ȥþ����������Ĥ���������ʢ���������з�̪�ı���̼��������Һ���Թ��У�Ѹ�ٷ�Ӧ�������������ݺͰ�ɫ�������Һ��dz���졣
��ͬѧ�Է�Ӧ�в����İ�ɫ�������������²²⣺
�²�1����ɫ���������Ϊ________________��
�²�2����ɫ���������ΪMgCO3��
�²�3����ɫ����������Ǽ�ʽ̼��þ[xMgCO3��yMg(OH)2]��
(2)��ƶ���ʵ��ȷ�����ﲢ��֤�²⣺
| ʵ����� | ʵ�� | ʵ������ | ���� |
| ʵ��� | ��ʵ������ռ����������ȼ | �ܰ���ȼ�ա���������ɫ���� | ������ɷ�Ϊ________ |
| ʵ��� | ��ȡʵ����еİ�ɫ�����ϴ�ӣ���������________ | ��________________ __________________ __________________ | ��ɫ��������ܺ���MgCO3 |
| ʵ��� | ȡʵ����еij���Һ�������м�������CaCl2ϡ��Һ | ������ɫ���� | ����Һ�д���________ |
(3)Ϊ��һ��ȷ��ʵ���IJ����ƶ���ʵ�鷽������ͼ��ʾ��

��ȡʵ��������ø�������İ�ɫ������22.6 g����ּ��������ٲ�������Ϊֹ����ʹ�ֽ����������ȫ������װ��A��B�С�ʵ���װ��A����1.8 g��װ��B����8.8 g����ȷ����ɫ������Ļ�ѧʽ��__________________________ ______________________________________________��
(4)���ϻ�ѧ����ͻ�ѧƽ���ƶ�ԭ������Mg�ͱ���NaHCO3��Һ��Ӧ�����������ݺͰ�ɫ�������ԭ��____________________________________��
��Ƴ������������ƹ��գ������ŷŵķ�ˮ�к��еľ綾CN-���ӣ����������ƹ�����������������Ƶķ�ˮʱ�����ڴ���TiO2�����£�����NaClO��CN-����������OCN-���������������¼�����NaClO������N2��CO2������������Ա���ܱ�ϵͳ������ͼװ�ý���ʵ�飬��֤��������������Ч�ԣ����ⶨCN-�������İٷ��ʡ���Ũ����CN-���ӵ���ˮ�����NaClO��Һ�Ļ��Һ��200mL������CN-��Ũ��Ϊ0.05mol��L-1��������У�������Ƥ����һ��ʱ�����Ƥ���ͻ�����ʹ��Һȫ���������У��رջ������ش��������⣺
��1�����з�Ӧ�����ӷ���ʽΪ ��
��2���������ɵ������N2��CO2�⣬���и�����HCl��Cl2�ȣ�����ʵ����ͨ���ⶨ������̼������ȷ����CN-�Ĵ���Ч��������м���ij����Լ���___________������ĸ��
| A������ʳ��ˮ | B������NaHCO3��Һ | C��ŨNaOH��Һ | D��Ũ���� |
װ�м�ʯ�ҵĸ���ܵ������� ��
��4������ʢ�к�Ca(OH)2 0.02mol��ʯ��ˮ����ʵ�������й�����0.82 g���������ʵ���в��CN-�������İٷ��ʵ��� �����ò��ֵ��ʵ�ʴ����İٷ������ƫ�ͣ����Ҫ˵�����ܵ�����һ��ԭ�� ��
��5�������һ�������ȷ�ȵĽ��飨Ҫ�пɲ����ԣ�����ʹ������ù��ڸ��ӣ� ��
ʵ�����Թ�ҵ̼��ƣ�������Na+��Al3+��Fe3+�����ʣ�Ϊԭ����ȡCaCl2��H2O��CaO2����Ҫ�������£�
��1�������Լ�X��������ҺpHΪ���Ի������Գ�ȥ��Һ��Al3+��Fe3+����������Ҫ�ɷ���___________���Լ�X����ѡ�����е�________�����ţ���
| A��CaO | B��CaCO3 | C��NH3��H2O | D��Ba(OH)2 |
��3����CaCl2��ȡCaO2�ķ�Ӧ�У��¶Ȳ���̫�ߵ�ԭ����_______________��
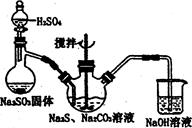
��4��������װ�òⶨ��ҵ̼��Ƶ���������
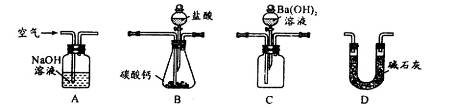
�ټ���װ��B���������õ�ʵ�������__________________________��
�ڰ�A��B��C��D˳�����ӣ�Ȼ���Aװ��ͨ�������Ŀ����_______________��
��װ��D������Ϊ______________________��
��ʵ��ʱ��ȷ��ȡ10.00g��ҵ̼���3�ݣ�����3�βⶨ�����BaCO3������ƽ������Ϊ17.73g������Ʒ��CaCO3����������Ϊ__________________��

 CH2=CH2����H2O������Ũ�������ǿ�����ԣ��丱�����ж�������Ͷ�����̼�ȡ�ijͬѧ����������Ϣ��ʵ��Ŀ��ѡ������ʵ��װ�����ʵ��̽��(ÿ��װ�ö������ɸ�)��
CH2=CH2����H2O������Ũ�������ǿ�����ԣ��丱�����ж�������Ͷ�����̼�ȡ�ijͬѧ����������Ϣ��ʵ��Ŀ��ѡ������ʵ��װ�����ʵ��̽��(ÿ��װ�ö������ɸ�)��