جâؤ؟ؤعبف
»¯ر§سëةْ²ْ،¢ةْ»î،¢ةç»لأـاذدà¹ط،£دآءذسذ¹طثµ·¨ضذ²»صب·µؤتا( )
A£®ذآؤـش´ئû³µµؤحئ¹مسëت¹سأسذضْسع¼ُةظ¹â»¯ر§رجخيµؤ²ْةْ
B£®2010ؤê12شآ10بصءھ؛د¹ْ؟²ہ¥ئّ؛ٍ±ن»¯´َ»ل£¬ئنض÷زھزéجâتا½عؤـ¼ُإإµؤµحج¼¾¼أ،£µحج¼¾¼أ¾حتازشµحؤـ؛ؤ،¢µحخغب¾،¢µحإإ·إخھ»ù´،µؤ¾¼أ·¢ص¹ؤ£ت½
C£®»ھزل؟ئر§¼ز¸كçûشع¹âدث´«تنذإد¢ءىسٍضذب،µأح»ئئذش³ة¾ح£¬¹âدثµؤض÷زھ³ة·ضتا¸ك´؟¶بµؤµ¥ضت¹è
D£®³¬¼¶²،¾ْNDM£1¼¸؛ُ¶شثùسذ؟¹ةْثط¶¼¾كسذ؟¹ز©ذش£¬ثہحِآت؛ـ¸ك،£خھ·ہض¹³¬¼¶²،¾ْµؤ¸ذب¾£¬زھ¼سا؟»·¾³،¢¸ِبثµؤخہةْ؛حدû¶¾£¬ئنضذدû¶¾¼ء³£ر،سأ؛¬آبدû¶¾¼ء،¢ث«رُث®،¢¾ئ¾«µبتتزثµؤخïضت
C
،¾½âخِ،؟¹âدثµؤض÷زھ³ة·ضتاSiO2،£

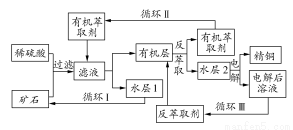
 شؤ¶ء؟ى³µدµءذ´ً°¸
شؤ¶ء؟ى³µدµءذ´ً°¸راآبثلؤئ(NaClO2)تاز»ضضضطزھµؤ؛¬آبدû¶¾¼ء£¬ض÷زھسأسعث®µؤدû¶¾زش¼°·ؤض¯ئ·µؤئ¯°×،£¹رُ»¯اâ·¨ةْ²ْراآبثلؤئµؤ²ظ×÷²½ضèبçدآ£؛
¢ظ½«آبثلؤئ(NaClO3)؛حرخثل¼سبëµ½ClO2·¢ةْئ÷ضذ£»
¢ع½«²ْةْµؤClO2ئّجهشعخب¶¨×°ضأضذسأث®خüتص؛َ£¬شظ¼سبëNaOH؛حث«رُث®£»
¢غشع¹ججه·ضہë×°ضأضذ½ّذذ·ضہ룬µأµ½راآبثلؤئ،£
زرضھNaClO2µؤبـ½â¶بثوخآ¶بة¸ك¶ّشِ´َ£¬تتµ±جُ¼دآ؟ة½ل¾§خِ³ِNaClO2،¤3H2O£¬ازNaClO2شع¼îذشجُ¼دآخب¶¨ذش½د¸ك،£تش»ط´ًدآءذختجâ£؛
(1)شعClO2·¢ةْئ÷ضذح¬ت±سذآبئّ²ْةْ£¬شٍشعClO2·¢ةْئ÷ضذ·¢ةْ·´س¦µؤ»¯ر§·½³جت½خھ ،£
(2)شعClO2خب¶¨×°ضأضذ£¬H2O2×÷ (ر،جîذٍ؛إ)،£
A£®رُ»¯¼ء
B£®»¹ش¼ء
C£®¼ب×÷رُ»¯¼ءسض×÷»¹ش¼ء
D£®¼ب²»×÷رُ»¯¼ءز²²»×÷»¹ش¼ء
(3)شعتµرéتزؤ£ؤâ،°¹ججه·ضہë×°ضأ،±ضذµؤ¼¼تُ£¬±طذë½ّذذµؤتµرé²ظ×÷تا (°´تµرéدب؛َث³ذٍجîذ´²ظ×÷´ْ؛إ)،£
A£®¹آث B£®¼سبب C£®صô·¢
D£®·ضز؛ E£®صôءَ F£®ہنب´
(4)¾²éشؤ×تءد؟ةضھ£¬µ±pH،ـ2.0ت±£¬ClO2-ؤـ±»I£حêب«»¹ش³ةCl££¬سû²â¶¨³ةئ·ضذNaClO2µؤ؛¬ء؟£¬دض½ّذذزشدآ²ظ×÷£؛
²½ضè¢ٌ | ³ئب،رùئ·Wgسع׶ذخئ؟ضذ£¬²¢µ÷½عpH،ـ2.0 |
²½ضè¢ٍ | دٍ׶ذخئ؟ضذ¼سبë×مء؟µؤKI¾§جه£¬²¢¼سبëةظء؟µؤض¸ت¾¼ء |
²½ضè¢َ | سأc mol،¤L£1µؤNa2S2O3بـز؛µخ¶¨£¬ةْ³ةI£؛حS4O62- |
¢ظ²½ضè¢ٍضذ·¢ةْ·´س¦µؤہë×س·½³جت½تا £¬²½ضè¢َضذ´ïµ½µخ¶¨ضصµمت±µؤدضدَتا ،£
¢عبôةدتِµخ¶¨²ظ×÷ضذسأب¥ءثV mL Na2S2O3بـز؛£¬شٍرùئ·ضذNaClO2µؤضتء؟·ضتخھ (سأ×ضؤ¸±يت¾)،£
¹¤زµةدةْ²ْءٍثلت±£¬ہûسأ´ك»¯رُ»¯·´س¦½«SO2×ھ»¯خھSO3تاز»¸ِ¹ط¼ü²½ضè،£ر¹ا؟¼°خآ¶ب¶شSO2×ھ»¯آتµؤس°دىبçدآ±ي(شءدئّ¸÷³ة·ضµؤجه»·ضتخھ£؛SO2 7%£¬O2 11%£¬N2 82%)£؛
ر¹ا؟/MPa ×ھ»¯آت/% خآ¶ب/،و | 0.1 | 0.5 | 1 | 10 |
400 | 99.2 | 99.6 | 99.7 | 99.9 |
500 | 93.5 | 96.9 | 97.8 | 99.3 |
600 | 73.7 | 85.8 | 89.5 | 96.4 |
(1)زرضھSO2µؤرُ»¯تا·إبب·´س¦£¬بç؛خہûسأ±يضذت¾فحئ¶د´ث½لآغ£؟________________________________________________________،£
(2)شع400،«500،وت±£¬SO2µؤ´ك»¯رُ»¯²ةسأ³£ر¹¶ّ²»تا¸كر¹£¬ض÷زھشزٍتا£؛__________________________________________،£
(3)ر،شٌتتزثµؤ´ك»¯¼ء£¬تا·ٌ؟ةزشجل¸كSO2µؤ×ھ»¯آت£؟________(جî،°تا،±»ٍ،°·ٌ،±)£¬تا·ٌ؟ةزششِ´َ¸أ·´س¦·إ³ِµؤببء؟£؟________(جî،°تا،±»ٍ،°·ٌ،±)،£
(4)خھجل¸كSO3خüتصآت£¬تµ¼تةْ²ْضذسأ________خüتصSO2،£
(5)زرضھ£؛2SO2(g)£«O2(g)=2SO3(g) ¦¤H£½£196.9 kJ،¤mol£1£¬¼ئثمأ؟ةْ²ْ1حٍ¶ض98%µؤءٍثلثùذèزھµؤSO3ضتء؟؛حسةSO2ةْ²ْصâذ©SO3ثù·إ³ِµؤببء؟،£