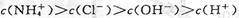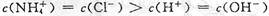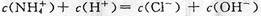��Ŀ����
(14��)���������У���NaCl����NaOH����NH3��H2O����CH3COOH��Һ����BaSO4����H2O����HCl;��H2SO4 ��CO2����ƾ���Һ������д��Żش�
����������ʵ��� ���Ƿǵ���ʵ��� ��
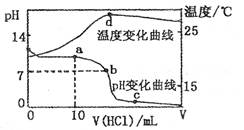
��pH��ȵĢڵ���Һ�͢۵���Һ�������ˮϡ����ͬ������pH����� ��
����pH��Ϊ2�Ģܡ��ߡ����������ʵ���Һ�����ʵ���Ũ�ȴ�С��˳��Ϊ �����ֱ������������кͺ������ʵ���NaOH����Һ����������Һ������ֱ�Ϊa��b��c����a��b��c�Ĵ�С��ϵ�� ��
(4)ij�¶�ʱ��0.01 mol?L-1�Ĵ�����Һ����ƽ�ⳣ��Ϊ1. 0��10 -8������ĵ���ƽ�ⳣ������ʽΪ ����ƽ��ʱ����Һ��������Ũ���� ���������Һ�м���һ����������ʱ�����볣�� �����仯�����ǡ���
��5����pH=1������ƽ���ֳ�2�ݣ�1�ݼ�����ˮ����1�ݼ�������������ʵ���Ũ����ͬ������NaOH��Һ��pH��������1���������ˮ��NaOH��Һ�������Ϊ ��
����������ʵ��� ���Ƿǵ���ʵ��� ��
��pH��ȵĢڵ���Һ�͢۵���Һ�������ˮϡ����ͬ������pH����� ��
����pH��Ϊ2�Ģܡ��ߡ����������ʵ���Һ�����ʵ���Ũ�ȴ�С��˳��Ϊ �����ֱ������������кͺ������ʵ���NaOH����Һ����������Һ������ֱ�Ϊa��b��c����a��b��c�Ĵ�С��ϵ�� ��
(4)ij�¶�ʱ��0.01 mol?L-1�Ĵ�����Һ����ƽ�ⳣ��Ϊ1. 0��10 -8������ĵ���ƽ�ⳣ������ʽΪ ����ƽ��ʱ����Һ��������Ũ���� ���������Һ�м���һ����������ʱ�����볣�� �����仯�����ǡ���
��5����pH=1������ƽ���ֳ�2�ݣ�1�ݼ�����ˮ����1�ݼ�������������ʵ���Ũ����ͬ������NaOH��Һ��pH��������1���������ˮ��NaOH��Һ�������Ϊ ��
�Ţۢޣ���Ƣۣ��� �ܢߢ࣬a��b=c��
(4) K= ��4.18��10 -4 mol?L-1 ���� ��5��11:1
��4.18��10 -4 mol?L-1 ���� ��5��11:1
(4) K=
 ��4.18��10 -4 mol?L-1 ���� ��5��11:1
��4.18��10 -4 mol?L-1 ���� ��5��11:1��1��������ʰ��������ᡢ���ˮ����������Σ���CH3COOH��Һ�ǻ����ʲ�����������ʣ�
��2��NaOH��ǿ���ȫ���룬NH3��H2O��������ֵ��룬�Ҵ���NH3��H2O�ĵ���ƽ�⣻��ˮϡ����ͬ������NH3��H2O�ĵ���ƽ�������ƶ�����[OH-]��NH3��H2O��NaOH�����ԣ�pH����Ǣ�NH3��H2O��
��3����CH3COOH������ ��HCl��һԪǿ�� ��H2SO4�Ƕ�Ԫǿ�ᣬ����PH��ȣ��������ƶ���H+Ũ����ȣ������ʵ���Ũ�ȴ�С��˳��Ϊ��CH3COOH(c��0.01mol.L-1)��HCl��c=0.01mol.L-1����H2SO4(c=0.005mol.L-1)��
��HCl��H2SO4�����ǣ�H+ + OH- = H2O
1 1
���кͺ������ʵ���NaOH����Һʱ��[H+]HClb=[H+]H2SO4c,��Ϊ[H+]HCl=[H+]H2SO4������b=c,
CH3COOH +NaOH= CH3COONa+H2O ,��c(CH3COOH)a =�����ʵ���NaOH����c(CH3COOH) ��[H+]HCl=[H+]H2SO4������a��b=c��
��4�� CH3COOH CH3COO-+H+
CH3COO-+H+
C0 0.01 0 0
��C x x x
C(ƽ��) 0.01-x x x
��x2/( 0.01-x)= 1.0��10 -8���ã�[H+]=" x" =4.18��10 -4 mol?L-1
ƽ�ⳣ�������¶�Ӱ�죬�ʼ���һ����������ʱ�����볣���������仯��
��5��PH��1�䵽2����H+Ũ����0.1mol?L-1�䵽0.01mol?L-1��
��һ�ݼ�ˮ��0.01mol?L-1=[0.1��V(HCl)]/[ V(HCl)+V(H2O)]���ã�V(H2O)=9V(HCl)��
�ڶ��ݼӼ0.01mol?L-1=[0.1��V(HCl)- 0.1��V(NaOH)]/[ V(HCl)+V(NaOH)]���ã�V(NaOH)=9/(11V),
���ԣ�V(H2O)��V(NaOH)= 11:1
��2��NaOH��ǿ���ȫ���룬NH3��H2O��������ֵ��룬�Ҵ���NH3��H2O�ĵ���ƽ�⣻��ˮϡ����ͬ������NH3��H2O�ĵ���ƽ�������ƶ�����[OH-]��NH3��H2O��NaOH�����ԣ�pH����Ǣ�NH3��H2O��
��3����CH3COOH������ ��HCl��һԪǿ�� ��H2SO4�Ƕ�Ԫǿ�ᣬ����PH��ȣ��������ƶ���H+Ũ����ȣ������ʵ���Ũ�ȴ�С��˳��Ϊ��CH3COOH(c��0.01mol.L-1)��HCl��c=0.01mol.L-1����H2SO4(c=0.005mol.L-1)��
��HCl��H2SO4�����ǣ�H+ + OH- = H2O
1 1
���кͺ������ʵ���NaOH����Һʱ��[H+]HClb=[H+]H2SO4c,��Ϊ[H+]HCl=[H+]H2SO4������b=c,
CH3COOH +NaOH= CH3COONa+H2O ,��c(CH3COOH)a =�����ʵ���NaOH����c(CH3COOH) ��[H+]HCl=[H+]H2SO4������a��b=c��
��4�� CH3COOH
 CH3COO-+H+
CH3COO-+H+ C0 0.01 0 0
��C x x x
C(ƽ��) 0.01-x x x
��x2/( 0.01-x)= 1.0��10 -8���ã�[H+]=" x" =4.18��10 -4 mol?L-1
ƽ�ⳣ�������¶�Ӱ�죬�ʼ���һ����������ʱ�����볣���������仯��
��5��PH��1�䵽2����H+Ũ����0.1mol?L-1�䵽0.01mol?L-1��
��һ�ݼ�ˮ��0.01mol?L-1=[0.1��V(HCl)]/[ V(HCl)+V(H2O)]���ã�V(H2O)=9V(HCl)��
�ڶ��ݼӼ0.01mol?L-1=[0.1��V(HCl)- 0.1��V(NaOH)]/[ V(HCl)+V(NaOH)]���ã�V(NaOH)=9/(11V),
���ԣ�V(H2O)��V(NaOH)= 11:1

��ϰ��ϵ�д�
 Сѧ�̲���ȫ���ϵ�д�
Сѧ�̲���ȫ���ϵ�д�
�����Ŀ
 H����F����25���£���20mL0.2mol?L-1��������еμ�0.2mol?L-1��NaOH��Һʱ����Һ��pH�仯��ͼ��ʾ��
H����F����25���£���20mL0.2mol?L-1��������еμ�0.2mol?L-1��NaOH��Һʱ����Һ��pH�仯��ͼ��ʾ��
 ���͵���ƽ�ⳣ����K��һ�������������������������ϡ��Һ�еĵ���������
���͵���ƽ�ⳣ����K��һ�������������������������ϡ��Һ�еĵ��������� ��
�� ԼΪ___ _%��
ԼΪ___ _%�� = Ka(HF)
= Ka(HF) 2H�� + S2��
2H�� + S2�� H3O++CO32-
H3O++CO32-