��Ŀ����
����A��B��C��D��E�����л�������������ת����ϵ��
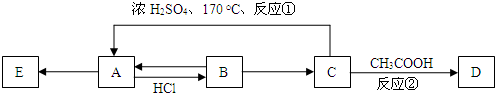
��֪��A����Է�������Ϊ28������E���ڸ߷��ӻ����
��������и��⣺
��1��д������B�Ľṹ��ʽ
��2��д������E�Ľṹ��ʽ
 ��
��
��3����Ӧ�ٵĻ�ѧ����ʽ
��4����Ӧ������
��5����Ӧ�ڵĻ�ѧ����ʽ
��6����Ӧ������

��֪��A����Է�������Ϊ28������E���ڸ߷��ӻ����
��������и��⣺
��1��д������B�Ľṹ��ʽ
CH3CH2Cl
CH3CH2Cl
����2��д������E�Ľṹ��ʽ


��3����Ӧ�ٵĻ�ѧ����ʽ
CH3CH2OH
CH2=CH2+H2O
| Ũ���� |
| 170�� |
CH3CH2OH
CH2=CH2+H2O
��| Ũ���� |
| 170�� |
��4����Ӧ������
��ȥ
��ȥ
��Ӧ����5����Ӧ�ڵĻ�ѧ����ʽ
CH3CH2OH+CH3COOH
CH3COOCH2CH3+H2O
| Ũ���� |
| �� |
CH3CH2OH+CH3COOH
CH3COOCH2CH3+H2O
��| Ũ���� |
| �� |
��6����Ӧ������
����
����
��Ӧ����������A��Է�������Ϊ28����AӦΪCH2=CH2����HCl�����ӳɷ�Ӧ����B��BΪCH3CH2Cl��C��Ũ���������¼��ȷ�����ȥ��Ӧ����A����֪CΪCH3CH2OH�������ᷢ��������Ӧ����DΪCH3COOCH3��EΪ����ϩ������л���Ľṹ�������Լ���ĿҪ��ɽ����⣮
����⣺��A��Է�������Ϊ28����AӦΪCH2=CH2����HCl�����ӳɷ�Ӧ����B��BΪCH3CH2Cl��C��Ũ���������¼��ȷ�����ȥ��Ӧ����A����֪CΪCH3CH2OH�������ᷢ��������Ӧ����DΪCH3COOCH3��EΪ����ϩ��
��1�������Ϸ�����֪BΪCH3CH2Cl��
�ʴ�Ϊ��CH3CH2Cl��
��2��EΪ����ϩ����ṹ��ʽΪ ��
��
�ʴ�Ϊ�� ��
��
��3����Ӧ��Ϊ�Ҵ�����ȥ��Ӧ����Ӧ�ķ���ʽΪCH3CH2OH
CH2=CH2+H2O��
�ʴ�Ϊ��CH3CH2OH
CH2=CH2+H2O��
��4����Ӧ��Ϊ�Ҵ�����ȥ��Ӧ��
�ʴ�Ϊ����ȥ��
��5����Ӧ��Ϊ�Ҵ��������������Ӧ������ʽΪCH3CH2OH+CH3COOH
CH3COOCH2CH3+H2O���ʴ�Ϊ��CH3CH2OH+CH3COOH
CH3COOCH2CH3+H2O��
��6����Ӧ��Ϊ�Ҵ��������������Ӧ��
�ʴ�Ϊ��������
��1�������Ϸ�����֪BΪCH3CH2Cl��
�ʴ�Ϊ��CH3CH2Cl��
��2��EΪ����ϩ����ṹ��ʽΪ
 ��
���ʴ�Ϊ��
 ��
����3����Ӧ��Ϊ�Ҵ�����ȥ��Ӧ����Ӧ�ķ���ʽΪCH3CH2OH
| Ũ���� |
| 170�� |
�ʴ�Ϊ��CH3CH2OH
| Ũ���� |
| 170�� |
��4����Ӧ��Ϊ�Ҵ�����ȥ��Ӧ��
�ʴ�Ϊ����ȥ��
��5����Ӧ��Ϊ�Ҵ��������������Ӧ������ʽΪCH3CH2OH+CH3COOH
| Ũ���� |
| �� |
| Ũ���� |
| �� |
��6����Ӧ��Ϊ�Ҵ��������������Ӧ��
�ʴ�Ϊ��������
���������⿼���л�����ƶϣ��ѶȲ���Ϊ�߿��������ͣ�������ѧ���ķ����������ƶ������Ŀ��飬ע������л�������ŵ������Լ���Ӧ��������Ϊ������Ĺؼ���

��ϰ��ϵ�д�
 �������Ͽ��㱾ϵ�д�
�������Ͽ��㱾ϵ�д�
�����Ŀ
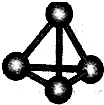 ����A��B��C��D��E��F���ֶ�����Ԫ�أ����ǵ�ԭ��������������D��E���⻯����ӹ��Ͷ���V�ͣ�A��B������������֮����C��������������ȣ�A�ֱܷ���B��C��D�γɵ���������ȵķ��ӣ���A��D���γɵĻ���������¾�ΪҺ̬��
����A��B��C��D��E��F���ֶ�����Ԫ�أ����ǵ�ԭ��������������D��E���⻯����ӹ��Ͷ���V�ͣ�A��B������������֮����C��������������ȣ�A�ֱܷ���B��C��D�γɵ���������ȵķ��ӣ���A��D���γɵĻ���������¾�ΪҺ̬��






