��Ŀ����
����Ŀ����ȥұ��ҵ�ŷ�������![]() �ķ����ж��֡�
�ķ����ж��֡�
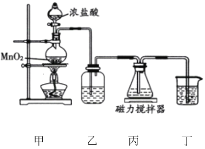
��1�����ñ�����Bunsen���Ȼ�ѧѭ������![]() ����������������Ӧ��ɣ�
����������������Ӧ��ɣ�
![]()
![]()
![]()
![]()
![]()
![]()
��![]()
![]() ________
________![]() ��
��
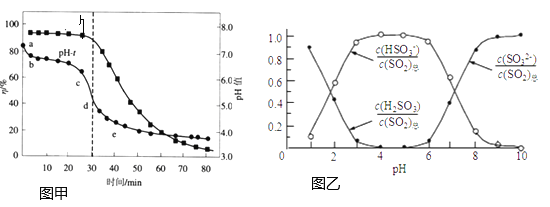
��2������п���շ�������![]() ����Һ�����������з��ѭ�������pH������Ч��
����Һ�����������з��ѭ�������pH������Ч��![]() ��ʱ��t�ı仯��ͼ�ף���Һ�в�������pH�Ĺ�ϵ��ͼ����ʾ��
��ʱ��t�ı仯��ͼ�ף���Һ�в�������pH�Ĺ�ϵ��ͼ����ʾ��
��Ϊ���![]() ������Ч��
������Ч��![]() ���ɲ�ȡ�Ĵ�ʩ�У���������Һ��
���ɲ�ȡ�Ĵ�ʩ�У���������Һ��![]() ������________��
������________��
��ͼ���е�![]() ����ab�η�������Ҫ��ѧ����ʽΪ________��
����ab�η�������Ҫ��ѧ����ʽΪ________��
��![]() ʱ����Һ
ʱ����Һ ________��
________��
��3����ͼ����ʾ�����ö��Ե缫��⺬![]() ����������S��
����������S��![]() ����ʵ�ַ��������á�
����ʵ�ַ��������á�

�������ĵ缫��ӦʽΪ________��
��ÿ������![]() �������������ϻ���S��
�������������ϻ���S��![]() �����ʵ����ֱ�Ϊ________��________��
�����ʵ����ֱ�Ϊ________��________��
���𰸡�![]() ������Һ��pH��6.8����
������Һ��pH��6.8���� ![]()
![]()
![]() 0.1mol 0.2mol
0.1mol 0.2mol
��������
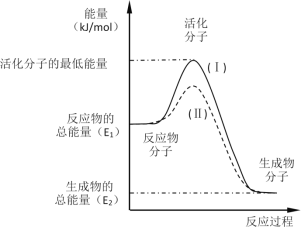
��1����֪����2H2(g)+O2(g)=2H2O(l) H1=-572kJ��mol-1
��2HI(g)=H2(g)+I2(g) H2=+10kJ��mol-1
��2H2SO4(l)=2SO2(g)+2H2O(g)+O2(g) H3=+462kJ��mol-1
����ݸ�˹���ɿ�֪-(����2+��+����2)���õ�SO2(g)+I2(g)+2H2O(l)=2HI(g)+H2SO4(l) H=+45kJ��mol-1��
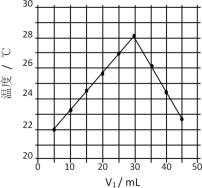
��2������������Һ��ZnO���������Գ�����ն������Ӷ��������Ч�ʣ��ʵ���ߵ�λʱ����������ѭ������������ʹ�������������գ��Ӷ�����˶������������Ч�ʣ��������ͼ���֪������Һ��pH��6.8����Ҳ���SO2������Ч�ʣ�
��ab����Һ��pH����4��6֮�䣬���ͼ2��֪����pH��������Һ����Ҫ����������������ӣ���ͼ1�е�pH-t����ab�η�������Ҫ��ѧ����ʽΪZnSO3+SO2+H2O=Zn(HSO3)2��
��pH=7ʱ������ͼ2��֪��Һ����������������������Ũ����ȣ�����ݵ���غ�2c(Zn2+)+c(H+)=c(OH-)+c(HSO3-)+2c(SO32-)��֪��Һ��![]() ��
��
��3��������SO2�õ�����ת��ΪS���ʣ���缫��ӦʽΪSO2+4H++4e-=S��+2H2O��
��������SO2ʧȥ����ת��Ϊ���ᣬ�ܷ�ӦʽΪ3SO2+2H2O![]() 2H2SO4+S����19.2g SO2�����ʵ�����0.3mol����������ϻ���S��H2SO4�����ʵ����ֱ�Ϊ0.1mol��0.2mol��
2H2SO4+S����19.2g SO2�����ʵ�����0.3mol����������ϻ���S��H2SO4�����ʵ����ֱ�Ϊ0.1mol��0.2mol��