��Ŀ����
ij�������������Al��(NH4)2SO4��MgCl2��AlCl3��CuCl2�е�һ�ֻ�����ɣ�
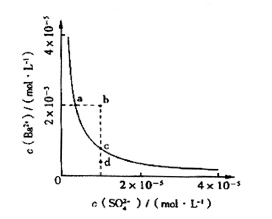
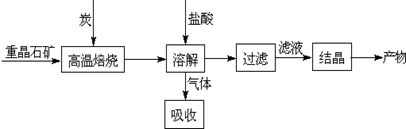
�ֶԸû����������ʵ�飬����������й�������ͼ��ʾ��������������ѻ���ɱ�״���µ��������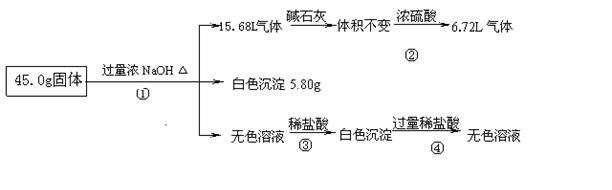
�ش��������⣺
�Ż�������Ƿ����CuCl2 ����ǡ�����
�ƻ�������Ƿ����(NH4)2SO4 ����ǡ���������ж������� ��
��д����Ӧ���е����ӷ���ʽ
��
(4)����ݼ������жϻ�������Ƿ���AlCl3��˵����ļ������ݣ���Ҫ��д������̣���
___________________________________________________________________________��
�ŷ�1�֣� ���ǣ�1�֣� ����ͨ��Ũ�������8.96L��(ֻҪ���������ټ��ɵ÷�) ��1�֣�
��3��H++OH-=H2O H2O+AlO2-+H+=Al(OH)3�� ����2�֣�
��4����������Ϣ���Ƶ�һ������Al��(NH4)2SO4��MgCl2�������ʣ���������֮�͵���41.3g��С�ڹ���������45.0g������ԭ�������һ������AlCl3����3�֣�
����������������ݷ�Ӧ����ͻ�ѧ�����ж����ʵ���ɣ����ݼ�������������Һ�õ���ɫ���������Ƴ����Ȼ�ͭ�����ɵ�����ͨ����ʯ��������䣬��ͨ��Ũ������٣�˵�������к��а�����������к�������泥��ۼ������ᣬ�Ⱥ͢��й������������Ʒ�Ӧ��Ȼ����������ƫ�������Ӧ�����ɰ�ɫ����������������ɫ����5.80gΪ������þ����������������Ȼ�þ������������ͨ��Ũ�������8.96L��Ϊ��������������������淋�������ʣ���6.72L����Ϊ��������������Һ��Ӧ���ɵ�������Ȼ�����Al��(NH4)2SO4��MgCl2��������֮��41.3g��С�ڹ���������45.0g������ԭ�������һ������AlCl3��
���㣺���⿼�����ʵ��ƶϡ����ӷ���ʽ����д����ѧ���㡣

 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д��ֱ���һ���Լ������������л�����������ʳ�ȥ��������Ϊ��������ʣ�
| ���� | �������Լ� | �й����ӷ���ʽ |
| ��1��HNO3(H2SO4) | | |
| ��2��Cu(Fe) | | |
| ��3��NaCl(Na2CO3) | | |
Cl2�Ƿ�֯��ҵ�г��õ�Ư����Na2S2O3����ΪƯ�ײ�ƥ��ġ����ȼ����� S2O32-��Cl2��Ӧ�IJ���֮һΪSO42һ������˵���У�������� ( )
| A���÷�Ӧ�е���������C12 |
| B��SO2����ˮ��Ư��ԭ����ͬ�����Կ���S02����֯��ҵ��Ư�� |
| C��������Ӧ�У�ÿ����1 mol SO42һ������ȥ2 mol C12 |
| D�����ݸ÷�Ӧ���жϻ�ԭ�ԣ�S2O32->C1�� |
�����й�������ԭ��Ӧ����������ȷ���� �� ��
| A��������ԭ��Ӧ�ı����ǵ��ӵ�ת�� |
| B����������ԭ��Ӧ�У�ʧȥ���ӵ����ʣ�һ����Ԫ�ػ��ϼ����� |
| C���϶���һ��Ԫ�ر���������һ��Ԫ�ر���ԭ |
| D���ڷ�Ӧ�в�һ������Ԫ�صĻ��ϼ۶������仯 |
 4CO��g��+BaS��s����H1=+571.2kJ?mol-1 ��
4CO��g��+BaS��s����H1=+571.2kJ?mol-1 ��