��Ŀ����
ʵ��������������480mL4.0 mol��L-1��NaOH��Һ������������Һ�ľ�����̻ش��������⣺
��1�����Ƹ���ҺӦѡ�� mL����ƿ��
��2����������ƽ��ȡ g����NaOH��
��3�����ƺõ�NaOH�������500mL�Ĵ��ձ��У���������ˮ���ò�������������ȫ�ܽ⡣����ȴ�����º��ձ��е���Һ�ò���������ת��������ƿ��
��4������������ˮϴ���ձ�2-3�Σ�����ÿ��ϴ�ӵ���Һ��ע�� �� ����ζ�����ƿ��ʹ��Һ��;��ȡ�
��5��������ƿ�м�������ˮ��ֱ��Һ����̶���Լ1-2����ʱ������ �μ�����ˮ��Һ����̶������С��Ǻ�ƿ����ҡ�ȡ�
��6�����ƺõ���Һ ����ܡ����ܡ������ڴ��������ƿ�С�
��7���������ػ����ʵ����ƫ�͵���
A������ʱ�۲�Һ������ �� B������ʱ�۲�Һ�温��
C��������NaOH��Һ�������ձ��� D������ƿ��ԭ������������ˮ
��ϰ��ϵ�д�
�����Ŀ


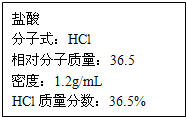 ��ͼΪʵ����ijŨ�����Լ�ƿ�ϵı�ǩ���Ը����й����ݻش��������⣺
��ͼΪʵ����ijŨ�����Լ�ƿ�ϵı�ǩ���Ը����й����ݻش��������⣺
 ʵ��������500mL 0.500mol?L-1��NaCl��Һ�������²������裺
ʵ��������500mL 0.500mol?L-1��NaCl��Һ�������²������裺