ΧβΡΩΡΎ»ί
ΓΨΧβΡΩΓΩΨέ¥ΉΥα““œ©θΞE «¥ΉΥα““œ©θΞDΒΡΨέΚœΈοΘ§”ΔΈΡΥθ–¥ΈΣPVAcΘ§÷ς“Σ”ΟΉςΆΩΝœΘ§“≤Ω…”ΟΉς÷Τ»ΓΨέ““œ©¥ΦΚΆΨέ““œ©¥ΦΥθ»©ΒΡ‘≠ΝœΓΘΙΛ“Β…œΩ…”Ο“‘œ¬Κœ≥…¬ΖœΏ÷Τ»ΓΘΚ
“―÷ΣΘΚAΒΡ’τΤχΟήΕ»œύΕ‘«βΤχΒΡΟήΕ» «14ΓΘ«κΜΊ¥πœ¬Ν–Έ ΧβΘΚ
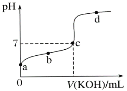
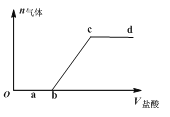
Θ®1Θ©AΒΡΫαΙΙΦρ ΫΈΣ__________Θ§DΒΡΖ÷Ή” ΫΈΣ__________Θ§B÷–ΙΌΡήΆ≈ΒΡΟϊ≥ΤΈΣ__________Θ§Ζ¥”ΠΔίΒΡΖ¥”Πάύ–ΆΈΣ__________ΓΘ
Θ®2Θ©Άξ»Ϊ»Φ…’Β»Έο÷ ΒΡΝΩΒΡAΓΔBΓΔCΘ§ΚΡ―θΝΩ¥”¥σΒΫ–ΓΒΡΥ≥–ρΈΣ__________Θ®”ΟΉ÷ΡΗ±μ ΨΘ©ΓΘ
Θ®3Θ©Ζ¥”ΠΔήΒΡΜ·―ßΖΫ≥Χ ΫΈΣ________________________________________ΓΘ
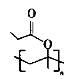
Θ®4Θ©D”–Εύ÷÷Ά§Ζ÷“λΙΙΧεΘ§Φ»ΡήΖΔ…ζΥ°ΫβΖ¥”ΠΘ§”÷ΡήΖΔ…ζ“χΨΒΖ¥”ΠΒΡDΒΡΆ§Ζ÷“λΙΙΧε”–__________÷÷Θ§Τδ÷–ΚΥ¥≈Ι≤’ώ«βΤΉ”–3ΉιΖεΘ§«“ΖεΟφΜΐ÷°±»ΈΣ1ΘΚ1ΘΚ4ΒΡΆ§Ζ÷“λΙΙΧεΒΡΫαΙΙΦρ ΫΈΣ__________ΓΘ
Θ®5Θ©ΗυΨί“―”–÷Σ Ε≤ΔΫαΚœœύΙΊ–≈œΔΘ§–¥≥ω“‘CH3CH=CH2ΚΆCH3CH2CH2OHΈΣ‘≠Νœ÷Τ±Η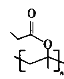 ΒΡΚœ≥…¬ΖœΏΝς≥ΧΆΦΘ®ΈόΜζ ‘ΦΝ»Έ”ΟΘ©ΘΚ____________________ΓΘ
ΒΡΚœ≥…¬ΖœΏΝς≥ΧΆΦΘ®ΈόΜζ ‘ΦΝ»Έ”ΟΘ©ΘΚ____________________ΓΘ
Κœ≥…¬ΖœΏΝς≥ΧΆΦ Ψάΐ»γœ¬ΘΚCH3CH2Br![]() CH3CH2OH
CH3CH2OH CH3COOCH2CH3
CH3COOCH2CH3
ΓΨ¥πΑΗΓΩCH2=CH2 C4H6O2 τ«Μυ Φ”ΨέΖ¥”Π A=B>C CH2=CH2+CH3COOH+O2 CH2=CH-OOCCH3+H2O 4
CH2=CH-OOCCH3+H2O 4 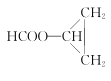 CH3CH2CH2OH
CH3CH2CH2OH![]() CH3CH2CHO
CH3CH2CHO![]() CH3CH2COOH
CH3CH2COOH![]() CH3CH2COOCΘ®CH3Θ©=CH2
CH3CH2COOCΘ®CH3Θ©=CH2![]()
ΓΨΫβΈωΓΩ
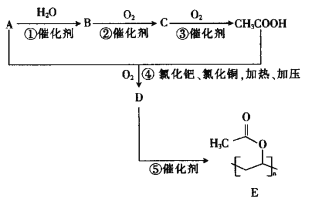
“―÷ΣAΒΡ’τΤχΟήΕ»œύΕ‘«βΤχΒΡΟήΕ» «14Θ§‘ρAΒΡΡΠΕϊ÷ ΝΩΈΣ28g/molΘ§AΓζBΓζCΓζCH3COOHΙΐ≥Χ÷–Θ§C‘≠Ή” ΐΡΩΈ¥±δΘ§‘ρAΒΡΖ÷Ή” ΫΈΣC2H4Θ§Φ¥““œ©ΘΜΩ…»ΖΕ®BΈΣ““¥ΦΘΜCΈΣ““»©ΘΜA”κ““ΥαΖΔ…ζ»Γ¥ζΖ¥”Π…ζ≥…DΘ§ΈΣCH2=CH-OOCCH3ΘΜDΖΔ…ζΦ”ΨέΖ¥”Π…ζ≥…EΓΘ
Θ®1Θ©AΈΣ““œ©Θ§ΤδΫαΙΙΦρ ΫΈΣCH2=CH2ΘΜDΈΣCH2=CH-OOCCH3Θ§Ζ÷Ή” ΫΈΣC4H6O2ΘΜBΈΣ““¥ΦΘ§Κ§”–ΒΡΙΌΡήΆ≈ΈΣτ«ΜυΘΜΖ¥”ΠΔίΈΣDΒΡΦ”ΨέΖ¥”ΠΘΜ
Θ®2Θ©AΓΔBΓΔCΖ÷±πΈΣ““œ©ΓΔ““¥ΦΓΔ““»©Θ§Ζ÷Ή” ΫΈΣC2H4ΓΔC2H6OΓΔC2H4OΘ§ΨυΈΣ1mol ±Θ§ΚΡ―θΝΩΖ÷±πΈΣ3molΓΔ3molΓΔ2.5molΘ§‘ρ”…¥σΒΫ–ΓΒΡΥ≥–ρΈΣA=B>CΘΜ
Θ®3Θ©Ζ¥”ΠΔήΈΣ““œ©ΓΔ““ΥαΓΔ―θΤχΖ¥”Π…ζ≥…CH2=CH-OOCCH3ΚΆΥ°Θ§ΖΫ≥Χ ΫΈΣCH2=CH2+CH3COOH+O2 CH2=CH-OOCCH3+H2OΘΜ
CH2=CH-OOCCH3+H2OΘΜ
Θ®4Θ©DΒΡΆ§Ζ÷“λΙΙΧε÷–Θ§Φ»ΡήΖΔ…ζΥ°ΫβΖ¥”ΠΘ§”÷ΡήΖΔ…ζ“χΨΒΖ¥”ΠΒΡ”–ΜζΈο÷–Κ§”–θΞΜυΘ§«“θΞΜυ‘Ύ“ΜΕΥΘ§Ά§Ζ÷“λΙΙΧε”–CH2=CH-CH2OOCHΓΔCH2=CΘ®CH3Θ©OOCHΓΔCH3CH=CHOOCHΓΔ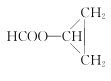 Θ§Ι≤ΦΤ4÷÷ΘΜΚΥ¥≈Ι≤’ώ«βΤΉ”–3ΉιΖεΘ§‘ρ”–2÷÷άύ–ΆΒΡ«β‘≠Ή”Θ§Ζ÷Ή”÷–÷Μ”–6Ηω«β‘≠Ή”Θ§ΖεΟφΜΐ÷°±»ΈΣ
Θ§Ι≤ΦΤ4÷÷ΘΜΚΥ¥≈Ι≤’ώ«βΤΉ”–3ΉιΖεΘ§‘ρ”–2÷÷άύ–ΆΒΡ«β‘≠Ή”Θ§Ζ÷Ή”÷–÷Μ”–6Ηω«β‘≠Ή”Θ§ΖεΟφΜΐ÷°±»ΈΣ![]() Θ§‘ρΗω ΐΈΣ1ΓΔ1ΓΔ4Θ§ΫαΙΙΦρ ΫΈΣ
Θ§‘ρΗω ΐΈΣ1ΓΔ1ΓΔ4Θ§ΫαΙΙΦρ ΫΈΣ ΘΜ
ΘΜ
Θ®5Θ©ΗυΨίΝς≥ΧΦΑΥυ―ß÷Σ ΕΘ§÷Τ±Η Θ§Ω…œ»÷Τ»Γ±ϊΥαΘ§‘Ό Ι±ϊΥα”κ±ϊœ©ΖΔ…ζ»Γ¥ζΖ¥”ΠΘ§ΉνΚσΦ”ΨέΘ§Φ¥Ω…Θ§Νς≥ΧΈΣCH3CH2CH2OH
Θ§Ω…œ»÷Τ»Γ±ϊΥαΘ§‘Ό Ι±ϊΥα”κ±ϊœ©ΖΔ…ζ»Γ¥ζΖ¥”ΠΘ§ΉνΚσΦ”ΨέΘ§Φ¥Ω…Θ§Νς≥ΧΈΣCH3CH2CH2OH![]() CH3CH2CHO
CH3CH2CHO![]() CH3CH2COOH
CH3CH2COOH![]() CH3CH2COOCΘ®CH3Θ©=CH2
CH3CH2COOCΘ®CH3Θ©=CH2![]()
 ΓΘ
ΓΘ