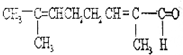��Ŀ����
5����ҵ����������������촼�� ������������������·�Ӧ���ã���100-106���·�Ӧ1.5h����������Һ��ˮϴ�Ӻ�5%��7%̼������Һ����pH=8��9����Һ������ˮ�Ȼ��ƣ������ռ�118��122����ֶ��ò�Ʒ��
������������������·�Ӧ���ã���100-106���·�Ӧ1.5h����������Һ��ˮϴ�Ӻ�5%��7%̼������Һ����pH=8��9����Һ������ˮ�Ȼ��ƣ������ռ�118��122����ֶ��ò�Ʒ����1����һ������ʱ���Һ��ˮϴ��Ŀ���dz�ȥ�����Ĵ���HBr��
��2��Ϊ����ϡ��̼������Һϴ�Ӷ������������ƣ��������ƿɵ������������ˮ�⣮
��3������ˮ�Ȼ��Ƶ�����������ˮ�����������������ķе���죮
��4���ù��̵Ļ�ѧ����ʽΪ��CH3��2CHCH2CH2OH+HBr$��_{100-106��}^{����}$��CH3��2CHCH2CH2Br+H2O��
���� ��1��ˮϴ�ɳ�ȥ�����Ĵ���HBr��
��2�����������ƿɵ������������ˮ�⣻
��3������ˮ�Ȼ��ƿ�����ˮ�����������������ķе���죻
��4�����촼��HBr����ȡ����Ӧ��������������ˮ��
��� �⣺��1����һ������ʱ���Һ��ˮϴ��Ŀ���dz�ȥ�����Ĵ���HBr���ʴ�Ϊ����ȥ�����Ĵ���HBr��
��2����ϡ��̼������Һϴ�Ӷ������������ƣ��������������ƿɵ������������ˮ�⣬�ʴ�Ϊ���������ƿɵ������������ˮ�⣻
��3������ˮ�Ȼ��Ƶ�����������ˮ�����������������ķе���죬����������룬�ʴ�Ϊ������ˮ�����������������ķе���죻
��4�����촼��HBr����ȡ����Ӧ��������������ˮ���÷�ӦΪ��CH3��2CHCH2CH2OH+HBr$��_{100-106��}^{����}$��CH3��2CHCH2CH2Br+H2O���ʴ�Ϊ����CH3��2CHCH2CH2OH+HBr$��_{100-106��}^{����}$��CH3��2CHCH2CH2Br+H2O��
���� ���⿼����������ᴿ��Ϊ��Ƶ���㣬�������ʵ����ʡ��������뷽����Ϊ���Ĺؼ������ط�����Ӧ�������Ŀ��飬��Ŀ�ѶȲ���

��ϰ��ϵ�д�
 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�
�����Ŀ