��Ŀ����
 ��08������ѧģ�⣩�����ܱ������еķ�Ӧ��N2��g��+3H2��g��
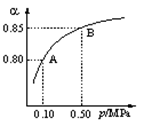
��08������ѧģ�⣩�����ܱ������еķ�Ӧ��N2��g��+3H2��g��![]() 2NH3��g������H ��0����673K��30MPa��n(NH3)��n(H2)��ʱ��仯�Ĺ�ϵ����ͼ��ʾ������������ȷ���ǣ���
2NH3��g������H ��0����673K��30MPa��n(NH3)��n(H2)��ʱ��仯�Ĺ�ϵ����ͼ��ʾ������������ȷ���ǣ���
A����a��������Ӧ���ʱȵ�b���Ĵ�
B���� c��������Ӧ���ʱ��淴Ӧ���ʴ�
C����d (t1ʱ��) ��n(N2)�ȵ� e (t2ʱ��) ����n(N2)��
D�������������䣬773K�·�Ӧ��t1ʱ�̣���ʱ����������ƽ����ľ��뽫Ҫ���

 Ӧ������ҵ��ϵ�д�
Ӧ������ҵ��ϵ�д���08������ѧģ�⣩��13�֣�ijѧУ��ѧ��ȤС��ͬѧ����֧С�Թ��зֱ����Ũ��Ϊ4mol��Lϡ����3mL�����ֱ�װ��0.3gNaHCO3��0.3gNa2CO3��ĩ��С����ֱ�������֧�Թܿڡ��������ڵ�NaHCO3��Na2CO3ͬʱ�����Թ��У��۲쵽�����������£�
(1)�Ƚ���֧�Թ��е����� ��
(2)���������ڵ�ѹǿ��ͬ�����Թܴ�С��ͬ�����С�������֮��ԼΪ (�����������)
(3)��ͬѧ���ִ����Թܣ�����ʢNaHCO3��ĩ���Թܱ��䣬��ʢNa2CO3���Թ��¶����ߡ��ɴ����ó���������״̬��Σ�NaHCO3��HCl��ӦΪ���ȷ�Ӧ����Na2CO3��HCl��ӦΪ���ȷ�Ӧ����ͬѧд���������Ȼ�ѧ����ʽ(���С�aq������ˮ�ϻ�����ϡ�͵ĺ���)��
HCO3��(aq)+H��(aq)=H2O(1)+CO2(g)����H>O��
CO32��(aq)+2H��(aq)=H2O(1)+CO2(g)����H<O��
�������۵ķ����Ƿ��ѧ? ��(��ǡ���)
(4)Ϊ�о���Ӧ�����Ȼ��Ƿ��ȣ���ͬѧ������������ʵ�顣(ÿ��ʵ��ƽ�������Σ�ȡƽ��ֵ)
�� �� | �Լ�l | �Լ�2 | ���ǰ�¶� | ��Ϻ��������¶� |
1 | 35mLˮ | 2.5gNaHCO3���� | 20�� | 18.5�� |
2 | 35mLˮ | 3.2gNa2CO3���� | 20�� | 24.3�� |
3 | 35mLϡ���� | ��2.5g NaHCO3 �ı�����Һ32.5ml | 20�� | 19�� |
4 | 35mLϡ���� | ��3.2g Na2CO3�ı�����23.1mL+10mLˮ | 20�� | 24.2�� |
5 | 35mLϡ���� | 2.5g NaHCO3���� | 20�� | 16.2�� |
6 | 35mLϡ���� | 3.2gNa2CO3���� | 20�� | 25.1�� |
���������д������ݣ�
�ٸ��о��������Ŀ���� ��
��ͨ������ʵ��ɵó��������ۣ�
a��NaHCO3���ܽ��� (�����ȡ����ȡ�)���̣�
b��Na2CO3���ܽ��� (�����ȡ����ȡ�)���̣�
c��NaHCO3�ı�����Һ������ķ�Ӧ�� (�����ȡ����ȡ�)��Ӧ��
d��Na2CO3�ı�����Һ������ķ�Ӧ�� (�����ȡ����ȡ�)��Ӧ��
e��NaHCO3������ϡ���ᷴӦ�ķ�Ӧ���� (��a��d����ĸ)����ЧӦ֮�͡�
��08������ѧģ�⣩(12��)ij����С���һЩ�������ʺͻ���������ʽ����о���
(1)�±�Ϊ�������Ȼ�ͭ��Һ��Ӧ��ʵ�鱨���һ���֣�
ʵ�鲽�� | ʵ������ |
����ĥ������Ƭ(����)����һ��Ũ�ȵ�CuCl2��Һ�� | �������ݣ��������ɵĺ�ɫ���壬��Һ��Ϊ��ɫ |
��Ӧ������������Һ���� |
|
��ɫ����������ˮϴ�Ӻ����ڳ�ʪ������ | һ��ʱ�������ɺ�ɫ��Ϊ��ɫ[������Ҫ�ɷ�ΪCu2(OH)2CO3] |
����Ӧ����д��ʵ���з�����Ӧ�Ļ�ѧ����ʽ��һ��(�����ӷ�Ӧ��ֻд���ӷ���ʽ)
�û���Ӧ��____________________________________��
���Ϸ�Ӧ��____________________________________��
(2)��ʯī���缫���������ʵ����������Һ�������������ݡ�������⣬��������������Һ�л��ɹ۲쵽��������_________________________________________�����ʹ���������ӷ���ʽ��_____________________________________
_________________________________________��
(3)��ҵ�Ͽ����������̿�(��Ҫ�ɷ�ΪMnO2)��Ӧ��ұ�������̡�
�����������̿�ұ���̵�ԭ����(�û�ѧ����ʽ��ʾ)______________________________��
��MnO2��H2O2�ֽⷴӦ������������������MnO2�����ữ���H2O2��Һ�У�MnO2�ܽ����Mn2�����÷�Ӧ�����ӷ���ʽ��______________________________________��
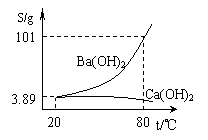 ��6�֣�ʵ������һ�ݻ�������̼������ʵ�̼�ᱵ��Ʒ�����벢�ᴿ̼�ᱵ��ʵ�鲽�����£������Ҫ����գ���ͼΪBa(OH)2��Ca(OH)2���ܽ�����ߣ���
��6�֣�ʵ������һ�ݻ�������̼������ʵ�̼�ᱵ��Ʒ�����벢�ᴿ̼�ᱵ��ʵ�鲽�����£������Ҫ����գ���ͼΪBa(OH)2��Ca(OH)2���ܽ�����ߣ���