��Ŀ����
17��ij�л���A����Է�������Ϊ128���ʣ���1����A��ֻ����C��HԪ�أ���A���ӽṹ�к��б�������A�ķ���ʽΪC10H8��
��2����A���������Һ˴Ź���������ֻ���������źŷ壬��A�����ֿ��ܵĽṹ��ʽΪ��CH3��3CCH2C��CH3��3��C��CH2CH3��4��
��3����A��ֻ����C��H��O����Ԫ�أ���A�����к���һ����Ԫ̼����һ���Ȼ������������������ţ���д��A�Ľṹ��ʽ
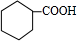 ��
��
���� ��1����2�������ķ���ʽΪCxHy��������̼ԭ�������Ŀ=$\frac{128}{12}$=10��8������ʽΪC10H8������1��Cԭ����Ҫ����12��Hԭ�ӣ�����ʽ��ΪC9H20��C9H20�DZ��������������������Hԭ����Ŀ��
��3��A�����к���һ����Ԫ̼����һ���Ȼ������������������ţ�6��̼ԭ��ʽ��Ϊ72��-COOH��ʽ��Ϊ45�����ò��෨��֪��ʣ����Ż�ԭ�ӵ���ʽ��Ϊ128-72-45=11���ʻ�ԭ11��Hԭ�ӣ�
��� �⣺��1�������ķ���ʽΪCxHy��������̼ԭ�������Ŀ=$\frac{128}{12}$=10��8������ʽΪC10H8������1��Cԭ����Ҫ����12��Hԭ�ӣ�����ʽ��ΪC9H20��C9H20�DZ��������������������Hԭ����Ŀ��
��A���ӽṹ�к��б�������A�ķ���ʽΪC10H8���ʴ�Ϊ��C10H8��
��2����A������������ʽ����ΪC9H20���˴Ź���������ֻ���������źŷ壬��A�����ֿ��ܵĽṹ��ʽΪ����CH3��3CCH2C��CH3��3��C��CH2CH3��4��
�ʴ�Ϊ����CH3��3CCH2C��CH3��3��C��CH2CH3��4��
��3����A�����к���һ����Ԫ̼����һ���Ȼ������������������ţ�6��̼ԭ��ʽ��Ϊ72��-COOH��ʽ��Ϊ45�����ò��෨��֪��ʣ����Ż�ԭ�ӵ���ʽ��Ϊ128-72-45=11���ʻ�ԭ11��Hԭ�ӣ�A�Ľṹ��ʽΪ ���ʴ�Ϊ��
���ʴ�Ϊ�� ��
��
���� ���⿼���л�����ƶϵȣ�ע���������෨����෨ȷ���л���Ľṹ���Ѷ��еȣ�

| ʵ��Ŀ�� | ʵ�鲽�� | |
| A | ̽�������Ի�ѧ��Ӧ���ʵ�Ӱ�� | ��H2O2��Һ�еμ�����FeCl3��Һ |
| B | ̽���Ҵ��ܷ���������Ӧ | ��ͭ˿�ھƾ��Ƽ��Ⱥ�����������ˮ�Ҵ��� |
| C | ̽��ʯ���ͷֽ�IJ��ﲻ�������� | ��ʯ���ͼ�ǿ�Ȳ���������ͨ��������Ȼ�̼��Һ�� |
| D | ��ȥ�����л��е���ϩ | �ѻ������ͨ�����Ը��������Һ |
| A�� | A | B�� | B | C�� | C | D�� | D |
��1��G��Q+NaCl
��2��Q+H20$\stackrel{���}{��}$X+H2
��3��Y+NaOH��G+Q+H2O
��4��Z+NaOH��X+Q+H2O
�����ֻ��������ȵĻ��ϼ��ɵ͵��ߵ�˳���ǣ�������
| A�� | Q G Z Y X | B�� | Z X G Y Q | C�� | G Y Z Q X | D�� | G Y Q Z X |
����ϩ�Ľṹ��ʽ��CH2CH2
��NH4Cl����ʽ��
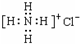
�۱���ȩ�ṹ��ʽ��
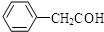
�ܱ�����ӵ����ģ��
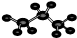
| A�� | �٢� | B�� | �ڢ� | C�� | �� | D�� | �ڢ� |
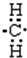
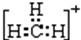
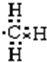
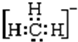
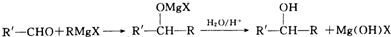
 �������
�������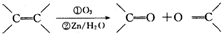
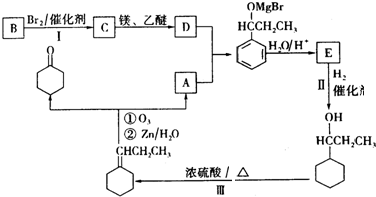
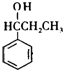 ��
��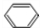 +Br2$\stackrel{����}{��}$
+Br2$\stackrel{����}{��}$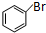 +HBr��
+HBr��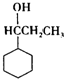 ��
��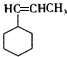 ��F��һ�������·����Ӿ۷�Ӧ�Ļ�ѧ����ʽ��n
��F��һ�������·����Ӿ۷�Ӧ�Ļ�ѧ����ʽ��n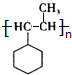 ��
��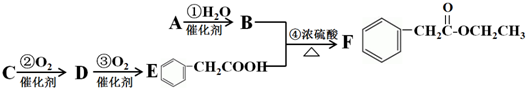
 ��
��