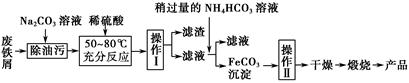
��Ŀ����
ij���Ͻ�(Ӳ��)�к���þ��ͭ���裬Ϊ�˲ⶨ�úϽ������ĺ����������������ʵ�飺
(1)ȡ��Ʒa g����ȡʱʹ�õ���������Ϊ ��
(2)����Ʒ��������ϡ�����У����ˣ���Һ����Ҫ���� �������к��� �����ܽ����ʱʹ�õ���Ҫ���������� ��
(3)����Һ�м������NaOH��Һ�����ˣ�д���ò��������йصĻ�ѧ����ʽ ��
(4)�ڵ�(3)������Һ��ͨ������CO2�����ˣ�������������ˮϴ�����κ�ɲ��������������ټ���Ϊֹ����ȴ�����������Ϊb g���йط�Ӧ�Ļ�ѧ����ʽΪ ��
(5)����Ʒ���������������ı���ʽΪ ��
(1)������ƽ
(2)MgCl2��AlCl3��Cu��Si��©�����ձ���������
(3)MgCl2��2NaOH=Mg(OH)2����2NaCl�� AlCl3��3NaOH=Al(OH)3����3NaCl�� Al(OH)3��NaOH=NaAlO2��2H2O
(4)NaAlO2��CO2��2H2O=NaHCO3��Al(OH)3�� 2Al(OH)3 Al2O3��3H2O
Al2O3��3H2O
(5) 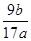 ��100%
��100%
����

 �Ķ��쳵ϵ�д�
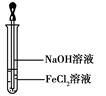
�Ķ��쳵ϵ�д���15�֣���FeCl3������Һ�ѳ�H2S��ķ�Һ��ͨ�����Ƶ�ѹ������������ijͬѧʹ��ʯī�缫���ڲ�ͬ��ѹ��x���µ��pH=1��0.1mol/LFeCl2��Һ���о���Һ������������¼���£�a��b��c������ѹֵ����
| ��� | ��ѹ/V | �������� | ������������ |
| I | x��a | �缫�������ֻ�ɫ�������ݲ��� | ��Fe3+����Cl2 |
| II | a��x��b | �缫�������ֻ�ɫ�������ݲ��� | ��Fe3+����Cl2 |
| III | b��x��0 | �����Ա仯 | ��Fe3+����Cl2 |
��2��I�У�Fe2+������ԭ�������Cl-�������ŵ磬���ɵ�Cl2��Fe2+������д���йط�Ӧ�ķ���ʽ_____��
��3����II�Ʋ⣬Fe3+������ԭ������Fe2+�������ŵ磬ԭ����Fe2+����_____�ԡ�
��4��II����δ����Cl2����Cl-�������Ƿ�ŵ������һ����֤�����pH=1��NaCl��Һ������ʵ�飬��¼���£�
| ��� | ��ѹ/V | �������� | ������������ |
| IV | a��x��c | �����Ա仯 | ��Cl2 |
| V | c��x��b | �����Ա仯 | ��Cl2 |
��IV�м��Cl2��ʵ�鷽��:____________________��
����II�Աȣ��ó��Ľ��ۣ�д�����㣩��___________________��
�п��Ľ���Na��¶�ڿ����У���仯�������£�
��1����Ӧ��ķ�Ӧ�����������仯�Ĺ�ϵ���£�
�� ��Ӧ�� ���� ����Ӧ������ȡ������ȡ������ж���������������
�� 1 mol Na(s)ȫ��������Na2O(s)���Ȼ�ѧ����ʽ����������
��2����Ӧ����Na2O��ˮ�ķ�Ӧ�������ĵ���ʽ�� ��
��3����ɫ��ĩΪNa2CO3����������ˮ����Ϊ0.1 mol/L Na2CO3��Һ������˵����ȷ���� ������ĸ����
| A�������¶ȣ���Һ��pH���� |
| B��c(OH��)��c (H��)��c (HCO3��)��2 c (H2CO3) |
| C����������NaOH���壬c (CO32�D)��c (Na��)������ |
| D��c (Na��) > c (CO32�D) > c (HCO3�D) > c(OH�D) > c (H��) |
�� �Ʊ�﮻��ã���ԭ�ӽṹ����ԭ��_______��
��ZEBRA �����һ���Ƶ�أ��ܷ�ӦΪNiCl2 + 2Na
 Ni + 2NaCl����������Ӧʽ��_____��
Ni + 2NaCl����������Ӧʽ��_____�� ��1�����µ�⼼���ܸ�Чʵ��CO2(g) + H2O(g) ="CO(g)" + H2(g) +O2(g) ������ԭ��ʾ��ͼ���£�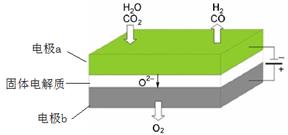
�ٵ缫b���� �����������ԭ������Ӧ��
��CO2�ڵ缫a�ŵ�ķ�Ӧʽ�� ��
��2����ҵ����ij����������Cu2O��Al2O3��Fe2O3��SiO2����ȡͭ�IJ����������£�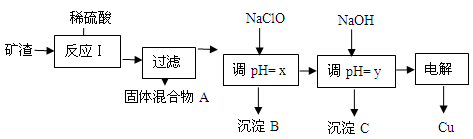
��֪�� Cu2O + 2H+ =" Cu" + Cu2+ + H2O
| ������ | Cu(OH)2 | Al(OH)3 | Fe(OH)3 | Fe(OH)2 |
| ��ʼ����pH | 5.4 | 4.0 | 1.1 | 5.8 |
| ������ȫpH | 6.7 | 5.2 | 3.2 | 8.8 |
�ٹ�������A�еijɷ��� ��
�ڷ�Ӧ����ɺ���Ԫ�صĴ�����ʽΪ ���������ӷ��ţ�
��д�����ɸ����ӵ����ӷ���ʽ ��
��x����ֵ��Χ��3.2��pH��4.0��y��Ӧ����ֵ��Χ�� ��
�����й���NaClO��pH��˵����ȷ���� ������ţ���
a������NaClO��ʹ��Һ��pH����
b��NaClO�ܵ���pH����Ҫԭ�������ڷ�����ӦClO��+ H+
 HClO��ClO������H+���Ӷ��ﵽ����pH��Ŀ��
HClO��ClO������H+���Ӷ��ﵽ����pH��Ŀ��c��NaClO�ܵ���pH����Ҫԭ��������NaClOˮ��ClO��+ H2O
 HClO+OH����OH������H+ ���Ӷ��ﵽ����pH��Ŀ��
HClO+OH����OH������H+ ���Ӷ��ﵽ����pH��Ŀ����ʵ����������������Ϊ20.0%��CuSO4��Һ�����Ƹ���Һ�����CuSO4��5H2O��H2O������֮��Ϊ ��
�ִ�ѭ������Ҫ���ۺϿ��ǻ�����Ⱦ�;���Ч�档�ߴ������������ִ����ӹ�ҵ�IJ��ϣ�
����������������������Ҫ�ɷ�ΪFe2O3��Fe3O4��FeO��SiO2��Ϊԭ���Ʊ��ߴ������������Ŧ�Fe2O3������������ʾ��ͼ��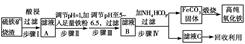
��1��������й���������������Ҫ�ɷ���________��
��2��������м������۵�Ŀ����________��
��3��������п�ѡ��________������Һ��pH��
| A��ϡ���� | B��˫��ˮ | C����ˮ | D�����������Һ |
��5������ҺC�л��յ���Ҫ���ʵ�һ����;��_________________________________��