��Ŀ����
��15�֣� A��B��C��X����ѧ��ѧ�������ʣ����ɶ�����Ԫ����ɣ�ת����ϵ��ͼ��������������ֲ�ͬ����ش�
��1����A��B��C�о���ͬһ�ֳ�������Ԫ�أ���Ԫ���� C������������ʽ���ڣ���A��C��ˮ��Һ��Ͽɵ�B�İ�ɫ��״������
�� A�к��еĽ���Ԫ�ص�ԭ�ӽṹʾ��ͼΪ ��
�� �ý���Ԫ�صĵ�����ij��ɫ�������ڸ����·�Ӧ�������ں������켰�����ƣ���֪��1mol�õ�����ȫ��Ӧ�����¶Ȼָ���298Kʱ��������QkJ����д���÷�Ӧ���Ȼ�ѧ��Ӧ����ʽΪ ��
��2����AΪ�л��75%��A��Һ����Ϊ�����������³�ѹ��B��C��Ϊ��ɫ���壬C��һ�ֳ�������������A�Ľṹ��ʽΪ�� ������ԭ���ԭ������B��X�ֱ�ͨ����A�Ƴɵ�����缫����20%��30%��KOH��Һ��Ϊ�������Һ��������ɻ�ѧ��Դ���õ�طŵ�ʱ�������缫��ӦʽΪ ��
��3����A��B��C����ɫ��Ӧ���ʻ�ɫ��ˮ��Һ��Ϊ���ԡ�
���û�ѧ����ʽ����C��Һ�ʼ��Ե�ԭ�� ��
�ڽ�4.48 L����״���£�Xͨ��100 mL3 mol/L A��ˮ��Һ����Һ������Ũ���ɴ�С��˳��Ϊ ��
����Ȼ���д���B��C��H2O��һ�������ᾧ���ɵĹ��塣ȡһ�����ù�������ˮ���100 mL��Һ�������Һ�н��������ӵ�Ũ��Ϊ0.5 mol/L����ȡ��ͬ�����Ĺ�����������أ�ʣ����������Ϊ___________________��
��15�֣�
��1������1�֣�
��2Al(s)+Fe2O3(s) Al2O3(s)+2Fe(s)����H����2QkJ/mol��3�֣�
��2��CH3CH2OH��1�֣� 2CO+8OH����4e����2CO32�� +4H2O��3�֣�
��3����CO32��+H2OOH��+ HCO3����2�֣�
��c(Na+)��c(HCO3��)��c(CO32��)��c(OH��)��c(H+)��2�֣�
��2.65 g��3�֣�
����:

 A��B��C��X����ѧ��ѧ1�г�����4�����ʣ����Ǿ��ɶ�����Ԫ����ɣ�ת����ϵ��ͼ��ʾ������������������1�ش��������⣺
A��B��C��X����ѧ��ѧ1�г�����4�����ʣ����Ǿ��ɶ�����Ԫ����ɣ�ת����ϵ��ͼ��ʾ������������������1�ش��������⣺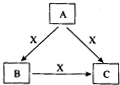 A��B��C��X����ѧ��ѧ�������ʣ�ת����ϵ��ͼ��ʾ����������²�ͬ����ش����⣺
A��B��C��X����ѧ��ѧ�������ʣ�ת����ϵ��ͼ��ʾ����������²�ͬ����ش����⣺ A��B��C��X����ѧ��ѧ�������ʣ����ɶ�����Ԫ����ɣ�ת����ϵ��ͼ��ʾ����������²�ͬ����ش�
A��B��C��X����ѧ��ѧ�������ʣ����ɶ�����Ԫ����ɣ�ת����ϵ��ͼ��ʾ����������²�ͬ����ش�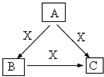 A��B��C��X����ѧ��ѧ�������ʣ����ɶ�����Ԫ����ɣ�ת����ϵ��ͼ��������������ֲ�ͬ����ش�
A��B��C��X����ѧ��ѧ�������ʣ����ɶ�����Ԫ����ɣ�ת����ϵ��ͼ��������������ֲ�ͬ����ش�
 A��B��C��X����ѧ��ѧ�г��������ʣ�����֮���ת����ϵ��ͼ��ʾ�����ֲ�������ȥ������ش��������⣺
A��B��C��X����ѧ��ѧ�г��������ʣ�����֮���ת����ϵ��ͼ��ʾ�����ֲ�������ȥ������ش��������⣺