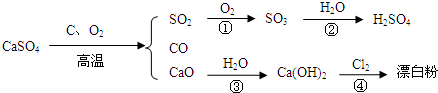
��Ŀ����
2��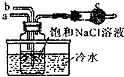 ��ˮ�����DZ����ˮ��Դ�������̲��ŷḻ�Ļ�ѧ��Դ����������֪��100����Ԫ���У�80%�����ں�ˮ���ҵ����û�ѧ֪ʶ�ش��������⣮
��ˮ�����DZ����ˮ��Դ�������̲��ŷḻ�Ļ�ѧ��Դ����������֪��100����Ԫ���У�80%�����ں�ˮ���ҵ����û�ѧ֪ʶ�ش��������⣮��1��2014��5�£��ҹ��㽭�غ�����������ġ��ೱ�����ೱ��������Ҫԭ��������ķ��������������賤��������ص�Ԫ����N��P��
��2��ij�о������гƺ�ˮ�е�������Ҫ��I-��̬���ڣ���ѧ�����ɺ�ˮ���ܽ�������ܰ�I-�������������ӷ���ʽ��ʾ��ͬѧ������4I-+O2+2H2O=2I2+4OH-��
��3��ij�������ĺ�ˮ��Ʒ�н�������Na+��K+��Mg2+��Cl-��SO42-����Na+��K+��Mg2+��Cl-��Ũ�Ⱦ�Ϊ0.02mol/L����c��SO42-��=0.03mol/L��
��4��ij�о�С����ʵ�����о���ȥ��ˮ��Ca2+��SO42-�����ӳ���������ΪBaCl2��Һ��Ba��OH��2��Na2CO3��Һ��Ȼ����ˣ�
��5����ͼ��ijС��ģ�⡰�����Ƽ����ȡNaHCO3�IJ���װ�ã����в�����ȷ����c
A��aͨ��CO2��Ȼ��bͨ��NH3��c�зż�ʯ��
B��bͨ��NH3��Ȼ��aͨ��CO2��c�зż�ʯ��
C��aͨ��NH3��Ȼ��bͨ��CO2��c�з�պϡ�������֬��
D��bͨ��CO2��Ȼ��aͨ��NH3��c�з�պϡ�������֬�ޣ�
���� ��1��N��PԪ�صĴ����ŷţ��ᵼ��ˮ��ĸ�Ӫ������
��2��������������ǿ��I2����O2�ܽ�I-����ΪI2����������ԭΪOH-��
��3��������Һһ��Ҫ�ʵ�������������
��4����ȥ��ˮ��Ca2+��SO42-�ֱ��ù�����Na2CO3��Һ��BaCl2��Һ���Լ��ļ���˳��Ҫ���㣺�����������µ��������ӣ�����������������ˣ���һ��Ҫ���׳�ȥ��
��5���������Ƽ����ȡNaHCO3��ԭ�� ���ڱ���ʳ��ˮ��ͨ�백���Ͷ�����̼�����õ�NaHCO3�ģ�����һʵ������У�����C02��ˮ�е��ܽ�Ƚ�С����NH3���ܽ�Ƚϴ�Ϊ��ֹ������b��ͨC02��a��ͨNH3������Ҫ��ʳ��ˮ��ͨNH3Ȼ����ͨC02������C02ͨ�����ˮ���ݳ�������ͨNH3ʱ��Һ��C02�����ͺ����ˣ������õ��IJ�ƷҲ���٣������ʵ���е�β����Ҫ��C02��NH3������NH3�Ի���Ӱ��ϴ�Ҫ���գ���NH3�Ǽ������壬������Cװ����Ҫװ�������ʣ��ݴ��ƶϣ�
��� �⣺��1������ϴ�Ӽ��Ĵ���ʹ�ã��ᵼ��N��PԪ�صĴ����ŷţ����ˮ��ĸ�Ӫ��������������ķ賤�������ೱ���ʴ�Ϊ��N��P��
��2��������������ǿ��I2����O2�ܽ�I-����ΪI2����������ԭΪOH-�����ӷ���ʽΪ��4I-+O2+2H2O=2I2+4OH-���ʴ�Ϊ��4I-+O2+2H2O=2I2+4OH-��
��3��������Һһ��Ҫ�ʵ����ԣ���֪��c��Na+��+c��K+��+2c��Mg2+��=c��Cl-��+2c��SO42-��������Na+��K+��Mg2+��Cl-��Ϊ0.02mol/L�����У�0.02mol/L+0.02mol/L+0.04mol/L=0.02mol/L+2c��SO42-������ã�c��SO42-��=0.03mol/L��
�ʴ�Ϊ��0.03��
��4����ȥ��ˮ��Ca2+��SO42-�ֱ��ù�����Na2CO3��Һ��BaCl2��Һ����Ba��OH��2����Ϊ���ܳ�ȥ������Ba2+����Na2CO3��Һһ��Ҫ��BaCl2��Ba��OH��2��Һ֮����룻����BaCl2��Ba��OH��2��Һ������BaSO4����������ʱ�����ȹ��ˣ���Ϊ��Һ�л���Ca2+������Ba2+������Na2CO3��Һ��������CaCO3��BaCO3��������ʱһ�����˳�ȥ���ɣ��ɽ�ʡʵ����Ʒ��ʱ�䣬
�ʴ�Ϊ��BaCl2��Һ��Ba��OH��2��
��4���������Ƽ����ȡNaHCO3��ԭ�����ڱ���ʳ��ˮ��ͨ�백���Ͷ�����̼�����õ�NaHCO3�ģ�����һʵ������У�����C02��ˮ�е��ܽ�Ƚ�С����NH3���ܽ�Ƚϴ�Ϊ��ֹ������b��ͨC02��a��ͨNH3������Ҫ��ʳ��ˮ��ͨNH3Ȼ����ͨC02������C02ͨ�����ˮ���ݳ�������ͨNH3ʱ��Һ��C02�����ͺ����ˣ������õ��IJ�ƷҲ���٣������ʵ���е�β����Ҫ��C02��NH3������NH3�Ի���Ӱ��ϴ�Ҫ���գ���NH3�Ǽ������壬������Cװ����Ҫװ�������ʣ��ݴ˿�֪A����B����C��ȷ��D����
��ѡC��
���� ���⿼����������ԭ��Ӧ����д����ˮ�ij����Լ������Լ��ļ���˳��͵缫��Ӧ����д��������ѧ����ʵ�����������������ʵ��ԭ���Ļ�����Ҫ��ʵ����ƵĿ�ѧ�ԡ���������ȷ���е��Ѷȣ�ע�ض�ʵ������Ŀ��飮

 ����ʦ��Сһ����ʦ������ҵϵ�д�
����ʦ��Сһ����ʦ������ҵϵ�д� ���100�ֵ�Ԫ�Ż�������ϵ�д�
���100�ֵ�Ԫ�Ż�������ϵ�д�| A�� | �������Ż�����ĭ��������� | |
| B�� | ʵ��̨�ϵľƾ��������Ż�������ʪĨ������ | |
| C�� | Ƥ������ŨHNO3�������ô���ˮ��ϴ������С�մ�ˮϴ�� | |
| D�� | ��������棬Ӧ��������۸��� |
| A�� | O3 | B�� | PCl3 | C�� | Na2O2 | D�� | NH4Cl |
| A�� | 0.01mol/L��������0.01 mol/L��NaOH��Һ�������Ϻ���Һ������ | |
| B�� | 0.01mol/L�Ĵ�����0.01 mol/L��NaOH��Һ�������Ϻ���Һ������ | |
| C�� | pH=2��������pH=12�İ�ˮ��Һ�������Ϻ���Һ�ʼ��� | |
| D�� | pH=2�Ĵ�����pH=12��NaOH��Һ�������Ϻ���Һ�ʼ��� |
| A�� | ��̼��ƺ�ϡ���ᷴӦ��ȡCO2��CaCO3+2H+�TCO2��+H2O+Ca2+ | |
| B�� | ��������ˮ�ķ�Ӧ��Na+H2O�TNa++OH-+H2�� | |
| C�� | ��������������Һ��Ӧ��Al+2OH-�TAlO2-+H2�� | |
| D�� | �����ۼ���ϡ�����У�2Fe+6H+�T2Fe3++3H2�� |
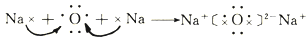
��
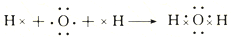
����˵������ȷ���ǣ�������
| A�� | �ٺ͢ڵı仯�����ж��е��ӵĵ�ʧ��ƫ�� | |
| B�� | �ٺ͢����õĻ������и�ԭ�Ӿ��ﵽ8�����ȶ��ṹ | |
| C�� | Na2O�к������Ӽ���H2O�к��м��Թ��ۼ� | |
| D�� | �ٺ͢ڹ�����ԭ�ӵ�������������Ӧ�Ļ������������ |
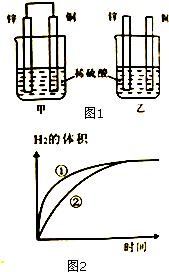 ��пƬ��ͭƬ����ͼ1��ʾ��ʽ����100mL��ͬŨ�ȵ�ϡ�����У�ij��ѧ��ȤС��̽����ѧ������ܵ��ת�����ش�
��пƬ��ͭƬ����ͼ1��ʾ��ʽ����100mL��ͬŨ�ȵ�ϡ�����У�ij��ѧ��ȤС��̽����ѧ������ܵ��ת�����ش�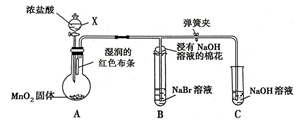 ��ͼ��һ����ȡ��������֤����ijЩ���ʵ�װ�ã����гֺͼ���װ��ʡ�ԣ�
��ͼ��һ����ȡ��������֤����ijЩ���ʵ�װ�ã����гֺͼ���װ��ʡ�ԣ�