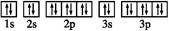��Ŀ����
[���ʽṹ������]
���ʽṹ�Ķ�����������ʹ�õIJ��Ͼ��ʷ׳ʡ�
��1��ͼ��ԭ���������������ֶ�����Ԫ�ص�һ������ʾ��ͼ��������Ԫ���� ����ͼ��Ԫ�ش��ţ���
��2���ڢ�����Ԫ����ɵĻ�������ۢ�����Ԫ����ɵĻ�����Ϊ�ȵ����壬д������ȵ������һ�����ӻ�ѧʽ
��3���Ҷ����������ᣬ������л���Ԫ��֮һ���ṹ��ʽΪ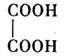 �����ƾ����д��� ����ͼ��Ԫ�ش��ţ���
�����ƾ����д��� ����ͼ��Ԫ�ش��ţ���
A������ B�Ǽ��Լ� C�� D���»��� E��� F���Ӽ�
��4��������ɵ������ԭ�������ǻ���̼��Ľṹʽ�ɱ���Ϊ ����̼�� �е�̼��ԭ�Ӷ���ͬһƽ�棬��̼ԭ�ӵ��ӻ������� ��
��5��PtCl4�Ͱ�ˮ��Ӧ�ɻ��PtCl4? 4NH3��PtCl4?4NH3��һ������100mL0.1mol?L-1PtCl4?4NH3 ��Һ�еμ�����AgNO3��Һ�ɲ���2.87g��ɫ�����������������ˮ�ĵ��뷽��ʽΪ ��
���ʽṹ�Ķ�����������ʹ�õIJ��Ͼ��ʷ׳ʡ�

��1��ͼ��ԭ���������������ֶ�����Ԫ�ص�һ������ʾ��ͼ��������Ԫ���� ����ͼ��Ԫ�ش��ţ���
��2���ڢ�����Ԫ����ɵĻ�������ۢ�����Ԫ����ɵĻ�����Ϊ�ȵ����壬д������ȵ������һ�����ӻ�ѧʽ
��3���Ҷ����������ᣬ������л���Ԫ��֮һ���ṹ��ʽΪ
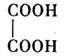 �����ƾ����д��� ����ͼ��Ԫ�ش��ţ���
�����ƾ����д��� ����ͼ��Ԫ�ش��ţ���A������ B�Ǽ��Լ� C�� D���»��� E��� F���Ӽ�
��4��������ɵ������ԭ�������ǻ���̼��Ľṹʽ�ɱ���Ϊ ����̼�� �е�̼��ԭ�Ӷ���ͬһƽ�棬��̼ԭ�ӵ��ӻ������� ��
��5��PtCl4�Ͱ�ˮ��Ӧ�ɻ��PtCl4? 4NH3��PtCl4?4NH3��һ������100mL0.1mol?L-1PtCl4?4NH3 ��Һ�еμ�����AgNO3��Һ�ɲ���2.87g��ɫ�����������������ˮ�ĵ��뷽��ʽΪ ��
��1����
��2��NO2��
��3��BCF
��4�� sp2
sp2
��5����PtCl2(NH3)4��Cl2 = ��PtCl2(NH3)4��2+ + 2Cl-
��2��NO2��
��3��BCF
��4��
 sp2
sp2��5����PtCl2(NH3)4��Cl2 = ��PtCl2(NH3)4��2+ + 2Cl-
�����������1��ͬ����Ԫ�صĵ�һ�����ܴ����������������ƣ����ڢ�A����A��Ԫ�ص�ԭ���������ӳ�ȫ���Ͱ���������ȶ��ṹ�������ϵͣ���һ�����ܽϴʢڡ��ֱ�ΪBe��N����ΪBԪ�أ���2��������Ϊ���Ӿ��壬�������Ӽ�������������к��зǼ��Լ���̼̼���������Լ���̼�����̼����������3��ͬ��Ԫ��ԭ�Ӽ۵����Ų���ͬ����4���������⺬��2���ǻ�����Ӧ�����ʻ����ʽṹʽΪ
 �����ӿռ�ṹΪƽ��ṹ��̼ԭ��Ϊsp2��
�����ӿռ�ṹΪƽ��ṹ��̼ԭ��Ϊsp2����5��n(Cl-)= n(AgCl)=2.87��143.5=0.02mol
n(PtCl4?4NH3)= 0.1��0.1=0.01mol������1mol�ܵ����2�������ӣ��������2�������ӣ���ѧʽΪPtCl2(NH3)4��Cl2�����뷽��ʽΪ��PtCl2(NH3)4��Cl2 = ��PtCl2(NH3)4��2+ + 2Cl-��

��ϰ��ϵ�д�
�����Ŀ
 ������ԭ�ӵ���ȼ�ϡ�
������ԭ�ӵ���ȼ�ϡ� ԭ�Ӻ��ں���������Ϊ
ԭ�Ӻ��ں���������Ϊ