��Ŀ����
��ͼ��Ԫ�����ڱ��Ŀ��
��1������Ԫ�����ڱ��ش��������⣺
�٣����ڱ��е�Ԫ�آݺ�Ԫ�آ�����������ˮ�������ǿ��˳����
���û�ѧʽ��ʾ����
�ڣ����ڱ��е�Ԫ�آܺ�Ԫ�آߵ��⻯����ۡ��е�ߵ�˳���� ���û�ѧʽ��ʾ����
�ۣ�������Ԫ�����ڱ���ȫ���ǽ���Ԫ�ص������� ��ȫ���Ƿǽ���Ԫ�ص������� ����д��ĸa��b��c��d����
a����A�� b����A�� c����A �� d����A��
��2����֪��Ԫ��λ�ڵ������ڣ�����ԭ�Ӱ뾶Ϊͬ���ڽ���Ԫ����ԭ�Ӱ뾶��С�ģ���д������������NaOH��Һ��Ӧ�����ӷ���ʽ �������� ��
��1����NaOH��Mg(OH)2��2�֣���HF��HCl��2�֣� �� b ��d ��ÿ��1�֣�2�֣�
��2��Al2O3 + 2OH��= 2AlO2��+ H2O ��2�֣�
���������������1���ٸ���Ԫ�����ڱ��е�λ�ÿ�֪��ΪNa����ΪMg��������������ˮ�������ǿ��˳����NaOH��Mg(OH)2��
�����ڱ��е�Ԫ�آ�ΪF��Ԫ�آ�ΪCl��HF���Ӽ��γ�����������Էе㣺HF��HCl��
�ۢ�A����Ԫ��ȫ�ǽ�������A����Ԫ��ȫ�Ƿǽ�����
��2����Ԫ��λ�ڵ������ڣ�����ԭ�Ӱ뾶Ϊͬ���ڽ���Ԫ����ԭ�Ӱ뾶��С�ģ����ΪAlԪ�أ�Al2O3��NaOH��Һ��Ӧ�����ӷ���ʽΪ��Al2O3 + 2OH��= 2AlO2��+ H2O��
���㣺���⿼��Ԫ�����ڱ���Ԫ�����ʵĵݱ��ԡ����ӷ���ʽ����д��

 ��ѧ�����ϵ�д�
��ѧ�����ϵ�д� �·Ƿ��̸����100��ϵ�д�
�·Ƿ��̸����100��ϵ�д���һ������I1��ָ��̬ԭ��X(g)ʧȥһ�����ӳ�Ϊ��̬������X+(g)���������������ʧȥ�ڶ����������������Ƶڶ������ܡ���ͼ�Dz���Ԫ��ԭ�ӵĵ�һ������I1��ԭ�������仯������ͼ���ش��������⣺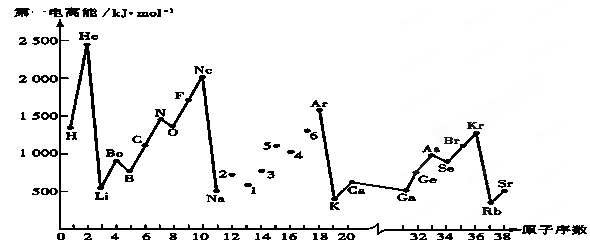
��1��ͬһ����Ԫ�صĵ�һ�����ܴ������������� ��������С�䣬��ͬ����ͬһ����Ԫ��ԭ�Ӵ��ϵ��µĵ�һ������I1�仯������______________��ϡ������ĵ�һ��������ͬ�����������ģ�ԭ����______________________________________________________________________��
��2�����������ͼ��ͬ����Ԫ�ص�һ�����ܵı仯���ɣ�������һЩ��������һ������ IIA��IIIA��VA��VIA: ��I1���룩��I1���𣩣�2�Ŵ���1�ţ�I1��������I1��������5�Ŵ���4�š�����ԭ���� ����3����֪2�ŵ�I1��738KJ/mol, ������I2 738KJ/mol�� I3 3��738KJ/mol�����������������4����֪5�ź�6��Ԫ�صĵ縺�Էֱ�Ϊ2.1��3.0����4��Ԫ�صĵ縺�Կ���Ϊ�� ��
| A��3.5 | B��1.8 | C��2.5 | D��4.0 |
�±�ΪԪ�����ڱ���һ���֣������Ԫ�آ٣����ڱ��е�λ�ã��û�ѧ����ش��������⣺
| �� ���� | IA | | 0 | |||||
| 1 | �� | ��A | ��A | ��A | ��A | ��A | ��A | |
| 2 | | | | �� | �� | �� | | |
| 3 | �� | | �� | �� | | | �� | |
��1���ܡ��ݡ���ԭ�Ӱ뾶�ɴ�С��˳��Ϊ________________������Ԫ�ط��ű�ʾ��
��2���ڡ��ۡ��ߵ���ۺ������������ǿ������˳����________�����û�ѧʽ��ʾ��
��3��ij�������ɢ١��ܡ�������Ԫ����ɣ�����ǿ�����ԣ��Ǻܺõ�Ư����ɱ��������д�����Ļ�ѧʽ��____________________��
��4����.���п���Ϊ�ȽϢ� �͢� �ǽ�����ǿ���������� ������ţ���
a����Ȼ���еĺ��� b���⻯����ȶ���
c�����������ˮ������Һ������ d���������ᷴӦʱʧȥ�ĵ�����
��.��ԭ�ӽṹ�ĽǶȽ��͢� �ķǽ�����ǿ�ڢ� ��ԭ���Ӳ�����ͬ���˵������ ���ڢ� ��ԭ�Ӱ뾶�� �ڣ�����ԭ�Ӻ˶��������ӵ��������� �ڣ��õ��������� ���ڢ� ��
��5������������۵����������ˮ������Һ��Ӧ�����ӷ���ʽ ��
���ֶ�����Ԫ��W��X��Y��Z��ԭ�����������������ϱ�����Ϣ�ش��������⡣
| | W | X | Y | Z |
| �ṹ������ | ������������������壬��ԭ�ӵ������������Ǵ�����������2�� | ����������Ӧ��ˮ����������̬�⻯�ﷴӦ�õ����ӻ����� | �������dz��������Ի����� | ��������������֮��Ϊ�� |
��1��Z������������Ҫ��;Ϊ ������Ȼ������ ����Ҫ�ɷ֡�
��2�������п���Ϊ�Ƚ�X��W�ǽ�����ǿ���������� ������ţ���
a����Ȼ���еĺ��� b���⻯����ȶ���
c�����������ˮ������Һ������ d���������ᷴӦʱʧȥ�ĵ�����
�ڴ�ԭ�ӽṹ�ĽǶȽ���X�ķǽ�����ǿ��W��ԭ���Ӳ�����ͬ���˵����X����W��ԭ�Ӱ뾶X W������ԭ�Ӻ˶��������ӵ�������X W���õ�������X����W ��
��3��Y����������X�����������ˮ������Һ��Ӧ�����ӷ���ʽ ��
 2X(g)����H ="-92.4" kJ��mol��1�����ʵ��Ĵ����ͺ��º�ѹ�����·�Ӧ������˵����ȷ���� ��
2X(g)����H ="-92.4" kJ��mol��1�����ʵ��Ĵ����ͺ��º�ѹ�����·�Ӧ������˵����ȷ���� ��