��Ŀ����
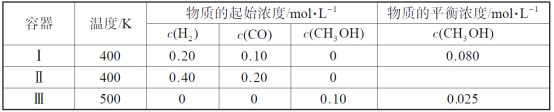
����Ŀ����(N2H4)�ڲ�ͬ�����·ֽ���ﲻͬ��200 ��ʱ��Cu����ֽ�Ļ�����ͼ����ʾ����֪200 ��ʱ��
��Ӧ��3N2H4(g)===N2(g)��4NH3(g) ��H1����32.9 kJ��mol��1
��Ӧ��N2H4(g)��H2(g)===2NH3(g) ��H2����41.8 kJ��mol��1
����˵���в���ȷ���� (�� ��)
A.ͼ��ʾ���̢١��ڶ��Ƿ��ȷ�Ӧ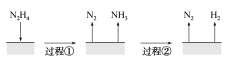
B.��Ӧ�����������ʾ��ͼ��ͼ��ʾ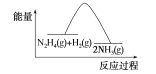
C.����3 mol N2H4(g)�еĻ�ѧ�����յ�����С���γ�1 mol N2(g)��4 mol NH3(g)�еĻ�ѧ���ͷŵ�����
D.200 ��ʱ���·ֽ����ɵ������������Ȼ�ѧ����ʽΪN2H4(g)===N2(g)��2H2(g)����H����50.7 kJ��mol��1
���𰸡�A
��������
���������֪�����⿼�鷴Ӧ�Ⱥ��ʱ�ļ��㣬���ø�˹���ɷ�����
A.���̢���N2H4�ֽ�����N2��NH3,��֪�Ȼ�ѧ����ʽI�С�HΪ��ֵ,����ͼʾ���̢�Ϊ���ȷ�Ӧ,���̢ڸ��ݸ�˹���ɣ�(I)2��(II)��N2H4(g)�TN2(g)+2H2(g)��H�T32.9kJmol12��(41.8kJmol1)=+50.7kJmol1��Ϊ���ȷ�Ӧ��A�����
B.��ӦII:N2H4(g)+H2(g)�T2NH3(g)��H2=41.8KJ/mol����ӦΪ���ȷ�Ӧ����Ӧ���������������B����ȷ��
C.��ӦI:3N2H4(g)�TN2(g)+4NH3(g)��Hl=32.9KJ/mol,��ӦΪ���ȷ�Ӧ,�Ͽ�3molN2H4(g)�еĻ�ѧ�����յ�����С���γ�1moIN2(g)��4molNH3(g)�еĻ�ѧ���ͷŵ�������C����ȷ��
D.���ݸ�˹���ɣ�(I)2��(II)��N2H4(g)�TN2(g)+2H2(g)��H�T32.9kJmol12��(41.8kJmol1)=+50.7kJmol1��D����ȷ��
��ѡA��