��Ŀ����
����Ŀ���±�ΪԪ�����ڱ���һ���֣���ش��й����⣺
��A | ��A | ��A | ��A | ��A | ��A | ��A | 0 | |
2 | �� | �� | ||||||
3 | �� | �� | �� | �� | �� | �� | ||
4 | �� | �� |
��1����������õĽ�����__________�������������뵼����ϵ���__________����ѧ�������ȶ�����__________ ����дԪ�ط��ţ���
��2���������γ��������������Ԫ����_________ ����дԪ�ط��ţ����ֱ�д����Ԫ�ص���������������������������ˮ���ﷴӦ�Ļ�ѧ����ʽ��_________________________��
��3����������ɵĻ���������__________���������ӻ��������������ۻ����������õ���ʽ��ʾ�û�������γɹ���________________________________�������⻯���к���__________���������Ӽ����������Թ��ۼ��������Ǽ��Թ��ۼ������ĵ���ʽ��__________��
��4�����������⻯���зе�ϸߵ���__________��д��ѧʽ��ԭ����_________��
��5�������һ��ʵ�飬�Ƚ��ߢ����������Ե�ǿ����________________________________��
���𰸡���1�� K�� Si ��Ar�� ��2��Al��2Al��OH��3��3H2SO4��Al2��SO4��3��6H2O��3�֣�
Al��OH��3��KOH��KAlO2��2H2O��3�֣� ��3�����ӻ����
![]() �����Թ��ۼ���
�����Թ��ۼ���![]()
��4��HBr����ΪHBr�ķ��Ӽ��������ϴ�
��5��ȡ��ɫ�廯�Ƶ�ˮ��Һ����������������ˮ����Һ��Ⱥ�ɫ
���������������������Ԫ�������ڱ��е����λ�ÿ�֪����N������Mg������Al����Si������S������Cl������Ar������K������Br��
��1��ͬ�������϶��½���������ǿ��ͬ�����������ҽ��������������������õĽ�����K�������������뵼����ϵ���Si����ѧ�������ȶ�����ϡ������Ar��
��2���������γ��������������Ԫ����Al����Ԫ�ص������������������������������������������������ˮ���ﷴӦ�Ļ�ѧ����ʽ�ֱ�Ϊ2Al��OH��3��3H2SO4��Al2��SO4��3��6H2O��Al��OH��3��KOH��KAlO2��2H2O��
��3����������ɵĻ��������廯þ���������ӻ�����õ���ʽ��ʾ�û�������γɹ��̿ɱ�ʾΪ![]() �������⻯���ǰ��������к��м��Թ��ۼ��������ĵ���ʽ��
�������⻯���ǰ��������к��м��Թ��ۼ��������ĵ���ʽ��![]() ��
��
��4���Ȼ�����廯���γɵľ�����Ƿ��Ӿ��壬��ΪHBr�ķ��Ӽ��������ϴ�����廯��ķе�ϸߡ�
��5�����õķǽ������û������õķǽ�������Ƚ��ߢ����������Ե�ǿ����ʵ������Ϊȡ��ɫ�廯�Ƶ�ˮ��Һ����������������ˮ����Һ��Ⱥ�ɫ��

 ��ĩ��ϰ���ϵ�д�
��ĩ��ϰ���ϵ�д� ����ѧ�䵥Ԫ������ĩר����100��ϵ�д�
����ѧ�䵥Ԫ������ĩר����100��ϵ�д� �Ƹ�360�ȶ����ܾ�ϵ�д�
�Ƹ�360�ȶ����ܾ�ϵ�д� ���⿼����Ԫ���Ծ�ϵ�д�
���⿼����Ԫ���Ծ�ϵ�д� ��У���˳�̾�ϵ�д�
��У���˳�̾�ϵ�д�����Ŀ��X��Y��WΪԭ���������ε����Ķ�����Ԫ�أ�X��Yͬ���壬Y��W����̬�⻯�������ͬ�ĵ�������һ�������X�ĵ���ֻ�������ԣ�
��1��д��ʵ������ȡW2��Ӧ�����ӷ���ʽ�� ��
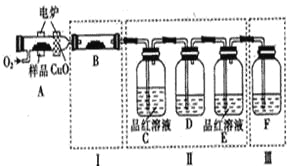
��2��ijС�������ͼ��ʾ��ʵ��װ�ã�ͼ�мгֺͼ���װ����ȥ��,�ֱ��о�YX2��W2�����ʡ�

���ֱ�ͨ��YX2��W2����װ��A�й۲쵽�������Ƿ���ͬ ��������ͬ����������ͬ��������װ��D��װ�������ۣ���ͨ��W2ʱD�й۲쵽������Ϊ ����װ��D��װ������������������ͨ��YX2ʱ����Kͨ������X2����ѧ����ʽΪ ��
����װ��B��װ��5.0mL1.0��10-3mol/L�ĵ�ˮ����ͨ������W2��ȫ��Ӧ��ת����5.0��10-5mol���ӣ���÷�Ӧ�Ļ�ѧ����ʽΪ ��
��3��ijͬѧ��������YX2ͨ��һ֧װ���Ȼ�����Һ���Թܣ�δ���������ɣ�����Թ��м������ ������ĸ�����Կ�����ɫ�������ɡ�
A����ˮ | B��ϡ���� | C��ϡ���� | D���Ȼ��� |
��4������Ԫ��Y��X���-2�����Z��Z��Y��X��������ΪY:X=4:3����W2�뺬Z����Һ��ȫ��Ӧ����dz��ɫ����������ȡ�ϲ���Һ���������Ȼ�����Һ���а�ɫ���������������ֳ�����������ȡ�д��W2��Z����Һ��ȫ��Ӧ����dz��ɫ���������ӷ���ʽ: ��