题目内容
【题目】现有21.6 g由CO和CO2组成的混合气体,在标准状况下其体积为13.44 L。回答下列问题:
(1)该混合气体的平均摩尔质量为________。
(2)混合气体中碳原子的质量为________。
(3)将混合气体依次通过如图所示装置,最后收集在气球中(实验在标准状况下测定)。
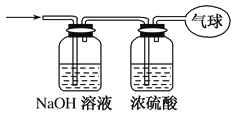
①气球中收集到的气体的摩尔质量为________。
②气球中收集到的气体中,电子总数为________(用NA表示阿伏加德罗常数的值)。
③气球的体积为________L。
【答案】 36 g·mol-1 7.2 g 28 g·mol-1 4.2NA 6.72
【解析】(1)标准状况下,该混合气体的物质的量为13.44÷22.4=0.6(mol),所以混合气体的平均摩尔质量M=m/n=21.6÷0.6=36 (g·mol-1),
(2)由第一问已知混合气体的物质的量为0.6mol,而 CO和CO2分子中均只含一个碳原子,故混合气体中的碳原子也为1mol,所以混合气体中碳原子的质量为0.6×12=7.2(g)
(3) CO和CO2组成的混合气体通过NaOH溶液后,CO2与NaOH反应被吸收 ,剩余的CO通过浓硫酸干燥,最后收集在气球中。设CO的物质的量为x ,则CO2的物质的量为0.6-x,列方程:28x+44(0.6-x)=21.6,解得x=0.3mol。
①气球中收集到的气体为纯净的CO,其摩尔质量为28 g·mol-1 ; ② CO的物质的量为0.3mol,所以电子总数为0.3×14NA=4.2NA ; ③标准状况下,0.3molCO的体积为0.3×22.4=6.72(L),所以气球的体积为6.72L。

 导学教程高中新课标系列答案
导学教程高中新课标系列答案 小学课时特训系列答案
小学课时特训系列答案【题目】为提纯下列物质(括号内物质为杂质),所选用的试剂和分离方法正确的是( )
物 质 | 除杂试剂 | 分离方法 | |
A | 硝酸铜溶液(硝酸银) | 铜粉 | 结晶 |
B | Cl2(HCl) | NaOH溶液 | 洗气 |
C | Br2(H2O) | 酒精 | 萃取 |
D | 铜粉(铁粉) | 稀盐酸 | 过滤 |
A.A
B.B
C.C
D.D