��Ŀ����
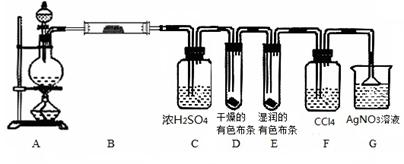
ij����С��������CuO��NH3��Ӧ���о�NH3��ij�����ʲ��ⶨ����ɣ����������ʵ��װ�ã��г�װ��δ����������ʵ�顣��ش��������⣺
��1������a������Ϊ________������b�п�ѡ����Լ�Ϊ________��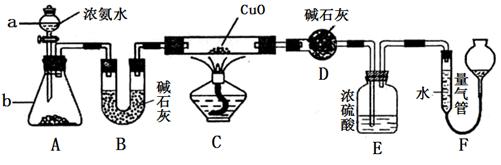
��2��ʵ�������������и������ʣ�������Ȫʵ�飬���ܳɹ����ǣ� ��
| A��Cl2�뱥��ʳ��ˮ | B��CO2 ��40%��NaOH��Һ |
| C��NH3�뱥��ʳ��ˮ | D��HCl��ˮ |
��4��Eװ����Ũ���������_______________________
��5��ʵ����ϣ�����ø����D����mg��װ��F�����������ΪnL(������ɱ�״�����������е������ԭ�Ӹ�����Ϊ________(�ú�m��n��ĸ�Ĵ���ʽ��ʾ)
��1����Һ©�� �����ƻ��������ƻ��ʯ��
��2��A
��3��3CuO + 2NH3 3Cu + N2�� + 3H2O
3Cu + N2�� + 3H2O
��4������δ��Ӧ�İ�������ֹF��ˮ��������D
��5��9n/11.2m
���������������1��ʵ�����г���Ũ��ˮ���Ȼ�Ũ��ˮ�μӵ����������ƻ����������ϣ����������ƻ��������Ƶ���ˮ�Լ��ܽ��������ʹ�����������2��������Ȫʵ��ɹ��Ĺؼ���������Һ�����ܽ�ȴ���������㹻���ѹǿ�Cl2�ڱ���ʳ��ˮ�ܽ���٣������γ�ѹǿ�������Ȫʵ�飬���ܳɹ�������ѡ����ԡ���3��NH3��CuO����ʱ����������ԭ��Ӧ�ķ���ʽ�ǣ�3CuO + 2NH3 3Cu + N2�� + 3H2O��4��Eװ����Ũ���������������δ��Ӧ�İ�������ֹF��ˮ��������D . ��5�������D����mg��������ˮ������������mg��װ��F�����������ΪnL������N2�ڱ�״�������nL��n(H2O):n(N2)=(mg��18g/mol):(nL��22.4L/mol)="11.2m:9n." �����е������ԭ�Ӹ�����Ϊ(9n��2): (11.2m��2)= 9n/11.2m
3Cu + N2�� + 3H2O��4��Eװ����Ũ���������������δ��Ӧ�İ�������ֹF��ˮ��������D . ��5�������D����mg��������ˮ������������mg��װ��F�����������ΪnL������N2�ڱ�״�������nL��n(H2O):n(N2)=(mg��18g/mol):(nL��22.4L/mol)="11.2m:9n." �����е������ԭ�Ӹ�����Ϊ(9n��2): (11.2m��2)= 9n/11.2m
���㣺����������ʶ��Ӧ�úͰ�����ʵ�����Ʒ������ʵ�֪ʶ��

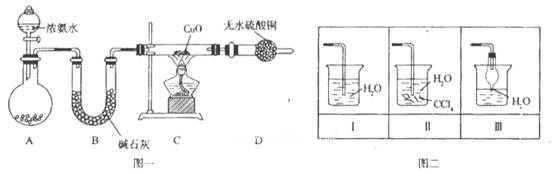
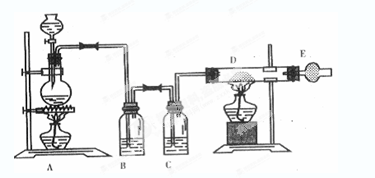
 ��ǰ����ϵ�д�
��ǰ����ϵ�д�������ͼ��ʾװ�ÿ������һϵ��ʵ�飨ͼ�мг�װ������ȥ��
��ش��������⣺
I����1������p��������_________________������װ��A��Ũ������������ƹ�����ȡSO2���壬��ͨ��װ��B��ɱ������ʵ�飬����д���пհף�
| B������� | �� | �� | �� |
| ��պ�Լ� | ʯ����Һ | Ʒ����Һ | ��ˮ����ɫ�� |
| ���� | | ��ɫ | |
| ����SO2������ | ˮ��Һ������ | | |
��2��д�����з�Ӧ�����ӷ���ʽ_________________________________________��
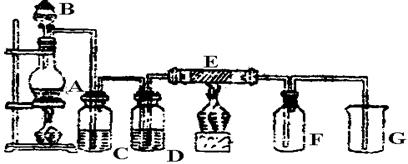
II������װ��A��Ũ������������ȡCl2���壬װ��B�е��Ĵ��������������´�������պ��FeCl2��Һ ��պ�е���KI��Һ ��պ��ʯ����Һ ��պ��Ʒ����Һ
��1��д���ٴ�������Ӧ�����ӷ���ʽ_______________________________________��
��2����ʵ������У��۴��ܹ۲쵽������___________________________________��
��3��д��װ��C�С����ն������塱���õ��Լ�_______________________________��
ij�����ĩ���п��ܺ���K2CO3��KNO3��NaNO2��K2SO3��Na2SO4��FeO��Fe2O3�е������֣�ijͬѧΪȷ���ù����ĩ�ijɷ֣�ȡ��������ʵ�飬ʵ����̼��������£� 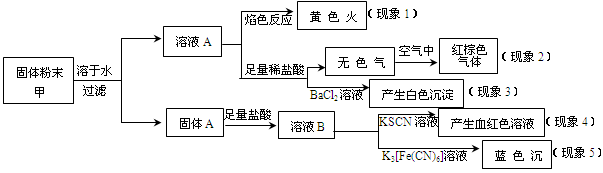
��ͬѧ�ó��Ľ�����ȷ����
| A����������1���Ƴ��ù����ĩ�к�����Ԫ�أ���������Ԫ�� |
| B����������2���Ƴ��ù����ĩ��һ������NaNO2 |
| C����������3���Ƴ��ù����ĩ��һ������Na2SO4 |
| D����������4������5���Ƴ��ù����ĩ��һ������FeO��Fe2O3 |
 5CaCl2 + Ca(ClO3)2 + 6H2O
5CaCl2 + Ca(ClO3)2 + 6H2O

 = ��
= ��

 CO2��+2SO2��+2H2O������ŨH2 S04����������� �������������ԭ������������0��2mol̼����ȫ��Ӧ��������H2S04�������� g������²���SO2�����Ϊ______________L��
CO2��+2SO2��+2H2O������ŨH2 S04����������� �������������ԭ������������0��2mol̼����ȫ��Ӧ��������H2S04�������� g������²���SO2�����Ϊ______________L��