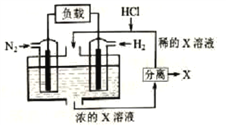
��Ŀ����
����Ŀ��ij��ѧʵ��С���ͬѧΪ̽���ͱȽ�SO2����ˮ��Ư���ԣ���������µ�ʵ��װ�á���ش��������⣺
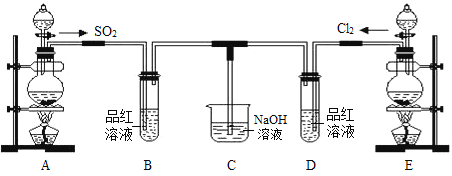
��1��ʵ������װ��E�Ʊ�Cl2���䷴Ӧ�Ļ�ѧ����ʽΪMnO2+4HCl(Ũ) ![]() MnCl2 + Cl2�� + 2H2O����������ѧ����ʽ��дΪ���ӷ���ʽ_______________________________��
MnCl2 + Cl2�� + 2H2O����������ѧ����ʽ��дΪ���ӷ���ʽ_______________________________��
��2���ٷ�Ӧ��ʼһ��ʱ��۲쵽B��D�����Թ��е�Ʒ����Һ����__________��������ѡ����ţ���ͬ����
A��Ʒ����Һ����ɫ B��Ʒ����Һ������ɫ
C��B��Ʒ����Һ��ɫ��D�в���ɫ D��D��Ʒ����Һ��ɫ��B�в���ɫ
��ֹͣͨ�����ٸ�B��D�����Թֱܷ���ȣ������Թ��е���Һ���ֵ���ɫ�ֱ�Ϊ________��
A����ɫ����ɫ B����ɫ����ɫ C����ɫ����ɫ D����ɫ����ɫ
��3��C�ձ�������������Һ��������_____________________________��д��Cl2ͨ��NaOH��Һ�еĻ�ѧ����ʽ_______________________________��
��4����һ��ʵ��С���ͬѧ��ΪSO2����ˮ����Ư���ԣ�����Ϻ��Ư���Կ϶����ǿ�����ǽ��Ƶõ�SO2��Cl2�����ʵ���֮��1�U1ͬʱͨ�뵽Ʒ����Һ�У����������ɫЧ�������������������������������ԭ�������ӷ���ʽ��ʾ��_______________________________________��
���𰸡�MnO2+4H++2Cl![]() Mn2+ +Cl2��+2H2O A D ���չ�����SO2��Cl2����ֹ��Ⱦ���� Cl2+2NaOH��NaCl+NaClO+H2O SO2+Cl2+2H2O��4H++2Cl+SO42
Mn2+ +Cl2��+2H2O A D ���չ�����SO2��Cl2����ֹ��Ⱦ���� Cl2+2NaOH��NaCl+NaClO+H2O SO2+Cl2+2H2O��4H++2Cl+SO42
��������
A�����Ʊ�SO2��ʵ�������������ƹ��������ᷴӦ��ȡSO2���壬�÷�ӦΪNa2SO3+H2SO4�TNa2SO4+SO2��+H2O��B���ڼ��������������ɣ�E��Ũ����Ͷ������̼����������Ʊ�������D���ڼ��������Ư���ԣ�C��������β����
��1��һ�𣺽�������ˮ��ǿ����ʲ��������ʽ����ɾ��ɾ������������ͬ�����ӣ��õ����ӷ���ʽ��
��2������ˮ������������Ư���ԣ����ܹ�ʹƷ����ɫ������ˮ�����ᣬ��������������ԣ���Ư��Ʒ�죬�����в������ԣ�SO2��Ư���п����ԣ�
��3���������Ƶ���������β����Cl2ͨ��NaOH��Һ�����Ȼ��ơ��������ơ�ˮ��
��4��SO2��Cl2��1��1ͨ�룬SO2��Cl2ǡ�÷�Ӧ����H2SO4��HCl�������ﶼ��Ư���ԣ�
��1��һ�𣺽�������ˮ��ǿ����ʲ��������ʽ����ɾ��ɾ������������ͬ�����ӣ����ӷ���ʽΪMnO2+4H++2Cl![]() Mn2+ +Cl2��+2H2O��
Mn2+ +Cl2��+2H2O��
��ȷ�𰸣�MnO2+4H++2Cl![]() Mn2+ +Cl2��+2H2O��
Mn2+ +Cl2��+2H2O��
��2���������Ͷ�����������Ʒ����Һ��ɫ������B��Dװ����Ʒ�춼��ɫ��
��ȷ�𰸣�A
�ڶ�������Ư������ʾ��в��ȶ��ԣ�����ʱ���ܱ�Ϊ��ɫ����������Ư���в������ԣ����Կ�����������B����Һ����ɫ��Ϊ��ɫ��D������������
��ȷ�𰸣�D��
��3���������Ƶ���������β����Cl2ͨ��NaOH��Һ�����Ȼ��ơ��������ơ�ˮ��
��ȷ�𰸣����չ�����SO2��Cl2����ֹ��Ⱦ���� Cl2+2NaOH��NaCl+NaClO+H2O��
��4��SO2��Cl2��1��1ͨ�룬SO2��Cl2ǡ�÷�Ӧ����H2SO4��HCl�������ﶼ��Ư���ԣ����ӷ���ʽSO2+Cl2+2H2O��4H++2Cl+SO42��
��ȷ�𰸣�SO2+Cl2+2H2O��4H++2Cl+SO42��

 ����˼ά�żӿ���ϵ�д�
����˼ά�żӿ���ϵ�д� �����Ծ�ϵ�д�
�����Ծ�ϵ�д� �ο�����������100��ϵ�д�
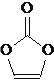
�ο�����������100��ϵ�д�����Ŀ����֪��![]() ��������ͼװ�����������ϳ�����ȩ������������£�
��������ͼװ�����������ϳ�����ȩ������������£�

���� | �е�/�� | �ܶ�/��g�� | ˮ���ܽ��� |
������ | 117.2 | 0.8109 | �� |
����ȩ | 75.7 | 0.8017 | �� |
����˵���У���ȷ���� �� ��
A. ���õĴ�����ȩ�м������������ƣ����������Ƿ���������
B. ���¶ȼ�1ʾ��Ϊ90��95�棬�¶ȼ�2ʾ����117.2������ʱ���ռ�����
C. ��Ӧ������������ﵹ���Һ©�����Է�ȥˮ�㣬������ȩ�ӷ�Һ©���Ͽڵ���
D. Ϊ�ӿ췴Ӧ��Ӧ���ữ��Na2Cr2O7��Һһ����ȫ��������������