��Ŀ����
4��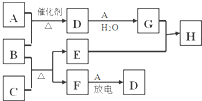 ��ͼ�漰����������Ԫ���У���һ��Ԫ���⣬�����Ϊ������Ԫ�أ���֪��A��FΪ��ɫ���嵥�ʣ�BΪ��ʹʪ���ɫʯ����ֽ���������壬CΪ��ɫ�����EΪ��ɫ�������ʣ�HΪ��ɫ��Һ�����ַ�Ӧ�IJ���δ�г�������ش��������⣺
��ͼ�漰����������Ԫ���У���һ��Ԫ���⣬�����Ϊ������Ԫ�أ���֪��A��FΪ��ɫ���嵥�ʣ�BΪ��ʹʪ���ɫʯ����ֽ���������壬CΪ��ɫ�����EΪ��ɫ�������ʣ�HΪ��ɫ��Һ�����ַ�Ӧ�IJ���δ�г�������ش��������⣺��1��C�Ļ�ѧʽΪCuO��
��2��F�ĵ���ʽΪ
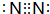 ��
����3��д��A��B��Ӧ�Ļ�ѧ����ʽ��4NH3+5O2$\frac{\underline{����}}{��}$4NO+6H2O��
��4��д��E��G��ϡ��Һ�����ӷ���ʽ��3Cu+8H++2NO3-=3Cu2++2NO��+4H2O��
��5��д��ʵ�����Ʊ�B�Ļ�ѧ����ʽ��Ca��OH��2+2NH4Cl$\frac{\underline{\;\;��\;\;}}{\;}$CaCl2+2NH3��+2H2O��
���� ת����ϵ����������Ԫ���У���һ��Ԫ���⣬�����Ϊ������Ԫ�أ�BΪ��ʹʪ���ɫʯ����ֽ���������壬��BΪNH3��EΪ��ɫ�������ʣ���EΪCu��������C��Ӧ�õ�Cu��F��CΪ��ɫ�������CΪCuO��FΪ��ɫ���嵥�ʣ�ֻ��ΪN2���÷�Ӧ����ˮ���ɣ�HΪ��ɫ��Һ����Cu��G��Ӧ�õ���H����ͭ���ӣ����������嵥��A��Ӧ�õ�D��D��������ˮ��Ӧ�õ�G������֪GΪHNO3��AΪO2��DΪNO���ݴ˽��
��� �⣺ת����ϵ����������Ԫ���У���һ��Ԫ���⣬�����Ϊ������Ԫ�أ�BΪ��ʹʪ���ɫʯ����ֽ���������壬��BΪNH3��EΪ��ɫ�������ʣ���EΪCu��������C��Ӧ�õ�Cu��F��CΪ��ɫ�������CΪCuO��FΪ��ɫ���嵥�ʣ�ֻ��ΪN2���÷�Ӧ����ˮ���ɣ�HΪ��ɫ��Һ����Cu��G��Ӧ�õ���H����ͭ���ӣ����������嵥��A��Ӧ�õ�D��D��������ˮ��Ӧ�õ�G������֪GΪHNO3��AΪO2��DΪNO��
��1��������������֪��C�Ļ�ѧʽΪCuO���ʴ�Ϊ��CuO��
��2��FΪN2������ʽΪ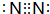 ���ʴ�Ϊ��
���ʴ�Ϊ��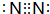 ��
��
��3��A��B��Ӧ�ǰ�����������������NO��ˮ����Ӧ��ѧ����ʽ��4NH3+5O2$\frac{\underline{����}}{��}$4NO+6H2O��
�ʴ�Ϊ��4NH3+5O2$\frac{\underline{����}}{��}$4NO+6H2O��
��4��Cu��ϡ���ᷴӦ��������ͭ��NO��ˮ����Ӧ���ӷ���ʽ��3Cu+8H++2NO3-=3Cu2++2NO��+4H2O��
�ʴ�Ϊ��3Cu+8H++2NO3-=3Cu2++2NO��+4H2O��
��5��ʵ�����Ʊ������Ļ�ѧ����ʽ��Ca��OH��2+2NH4Cl$\frac{\underline{\;\;��\;\;}}{\;}$CaCl2+2NH3��+2H2O��
�ʴ�Ϊ��Ca��OH��2+2NH4Cl$\frac{\underline{\;\;��\;\;}}{\;}$CaCl2+2NH3��+2H2O��
���� ���⿼��������ƶϣ����ʵ�Ԫ�����ƶ�ͻ�ƿڣ��ٽ��ת����ϵ�ƶϣ���Ҫѧ����������Ԫ�ػ������������ת�����Ѷ��еȣ�

 ��������һ���þ�ϵ�д�
��������һ���þ�ϵ�д� Сѧ��10����Ӧ����ϵ�д�
Сѧ��10����Ӧ����ϵ�д�| A�� | ���÷�Һ©������������Ȼ�̼ | |
| B�� | CH3COOCH3�˴Ź���������ֻ����һ���ź� | |
| C�� | �Ҵ���CH3CH2OH���Ͷ����ѣ�CH3-O-CH3����Ϊ̼���칹�� | |
| D�� | �������ӳɵõ�2��3-���������ϩ���ṹʽ��5�� |
| A�� | CO��NO���������Ѫ�쵰��ϣ�ʹ���ж� | |
| B�� | CaO����SO2��Ӧ��������ҵ����������� | |
| C�� | �⻯ѧ������������������� | |
| D�� | CO2��NO2��SO2���ᵼ��������γ� |
| A�� | NH4+ Na+ OH- | B�� | Na+ H+ SO32- | ||
| C�� | K+ NO3- Cl- | D�� | Ca2+ NO3- CO32- |
| A�� | ��ϩ�Ľṹ��ʽ��CH2CH2 | B�� | ��ϩ�ķ���ʽ��C2H4 | ||
| C�� | ����Ľṹ��ʽ��C2H4O2 | D�� | ������ӵ����ģ���� 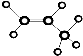 |
| A�� | Ũ���� | B�� | Ũ���� | C�� | ʳ�� | D�� | �������� |
| A�� | 0.2mol/L | B�� | 0.05mol/L | C�� | 0.45mol/L | D�� | 0.225mol/L |
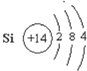 ��D��Ԫ�����ڱ��е�λ�õ�3���ڣ���VIA�壮
��D��Ԫ�����ڱ��е�λ�õ�3���ڣ���VIA�壮 ��
�� ���ӷ�Һ©���Ͽڵ������ϲ�Һ���DZ���
���ӷ�Һ©���Ͽڵ������ϲ�Һ���DZ���