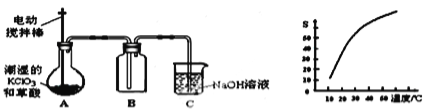
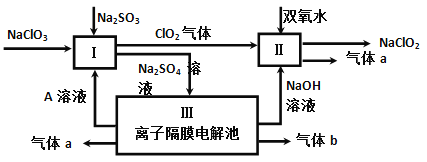
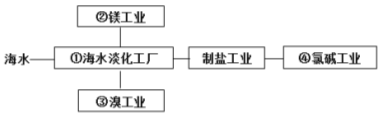
��Ŀ����
����Ŀ��ij��Һ��ֻ���ܺ������������еļ��֣�K+��NO![]() ��SO
��SO![]() ��NH
��NH![]() ��CO
��CO![]() ����������Һ��������H+��OH������ȡ200mL����Һ����Ϊ���ȷݽ�������ʵ�飺
����������Һ��������H+��OH������ȡ200mL����Һ����Ϊ���ȷݽ�������ʵ�飺
ʵ��1����һ�ݼ����������ռ���ȣ������������ڱ�״����Ϊ224mL��
ʵ��2���ڶ����ȼ������������ᣬ�������ټ���������BaCl2��Һ���ù���2.33g��
����˵����ȷ����
A. ����Һ�п��ܺ�K+
B. ����Һ�п϶�����NO![]() ��SO
��SO![]() ��NH
��NH![]() ��CO
��CO![]()
C. ����Һ��һ������NO![]()
D. ����Һ��һ����K+����c(K+)��0.1mol/L
���𰸡�D
�������������������1����һ�ݼ����������ռ���ȣ���NH4++OH-�TNH3��+H2O���������״����Ϊ224mL���壬֤������NH4+�������ʵ���Ϊ0.01mol����2���ڶ����ȼ������������ᣬ��������һ��������CO32-���ټ�������BaCl2��Һ���ù���2.33g��֤��һ������SO42-�������ʵ���Ϊ��n=m/M=2.33g��233g/mol=0.01mol��������Һ�еĵ���غ㣬��Һ�ʵ����ԣ���һ�����м����ӣ��Ҽ����ӵ�Ũ����(0.01mol��20.01mol)/0.1L=0.1mol/L�����Ը���Һ�п϶�����NH4+��SO42-��K+�����K+�����ʵ�������0.01mol����NO3-�����K+�����ʵ�������0.01mol����Ӧ����NO3-��A��������֪һ�����м����ӣ���A����B�������ж�̼�������һ�������ڣ���������ӿ��ܴ��ڣ���B����C����������ж���������ӿ��ܺ��У���C����D�������ƶϣ�һ������K+��c��K+����0.1 mol/L����D��ȷ����ѡD��

 ABC����ȫ�ž�ϵ�д�
ABC����ȫ�ž�ϵ�д�