��Ŀ����
��֪Ksp(AgCl)=1.8��10-10��Ksp(AgI)=1.0��10-16�����й���������֮��ת����˵���д������( )
| A��AgCl������ˮ������ת��ΪAgI |
| B�������������Ksp���Խ���������Խ����ת��Ϊ�����ܵ����� |
| C��AgI��AgCl��������ˮ������AgCl����ת��ΪAgI |
D�������£�AgCl��Ҫ��NaI��Һ�п�ʼת��ΪAgI����NaI��Ũ�ȱ��벻����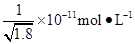 |
A
����

 ���Ӣ��������ϵ�д�
���Ӣ��������ϵ�д������£������йش�����Һ�������в���ȷ����(����)
| A��pH��5.6��CH3COOH��CH3COONa�����Һ�У�c(Na��)��c(CH3COO��) |
| B��Ũ�Ⱦ�Ϊ0.1 mol��L��1��CH3COOH��CH3COONa��Һ�������Ϻ�c(CH3COO��)��c(CH3COOH)��2[c(H��)��c(OH��)] |
| C����pH��a�Ĵ���ϡ��ΪpH��a��1�Ĺ����У�c(CH3COOH)/c(H��)��С |
| D�������pH��a�Ĵ�����pH��b��NaOH��Һǡ���к�ʱ��a��b��14 |
�����£���һ��Ũ�ȵ�����ʹ����ˮϡ�ͣ���Һ�ĵ�����������Һ����仯��������ͼ��ʾ���ж�����˵���У���ȷ���ǣ� ��
| A������Һϡ��ǰ��Ũ����ͬ |
| B��a��b��c������Һ��pH�ɴ�С˳��Ϊa��b��c |
| C��a���KWֵ��b���KWֵ�� |
| D��a��ˮ�����c(H��)����c��ˮ�����c(H��) |
����ѪҺ�������Ҫ�����ƽ�⣺ ��ʹ����ѪҺpH������7.35��7.45������ͻᷢ�����ж�����ж�����pH��c(HCO3-)��c(H2CO3)�仯��ϵ���±���
��ʹ����ѪҺpH������7.35��7.45������ͻᷢ�����ж�����ж�����pH��c(HCO3-)��c(H2CO3)�仯��ϵ���±���
| c(HCO3-)��c(H2CO3) | 1.0 | 17.8 | 20.0 | 22.4 |
| pH | 6.10 | 7.35 | 7.40 | 7.45 |
����˵������ȷ���ǣ� ��
A����������ѪҺ�У�HCO3-��ˮ��̶ȴ��ڵ���̶�
B������ѪҺ���ж�ʱ����ע��NaHCO3��Һ����
C��pH��7.00��ѪҺ�У�c(H2CO3)��c(HCO3-)
D��pH��7.40��ѪҺ�У�HCO3-��ˮ��̶�һ������H2CO3�ĵ���̶�
��֪25��ʱ��CaSO4��ˮ�еij����ܽ�ƽ��������ͼ��ʾ����100 mL�������µ�CaSO4������Һ�м���400 mL 0.01 mol��L��1Na2SO4��Һ����Դ˹��̵�����������ȷ����(����)
| A����Һ������CaSO4������������Һ��c(SO42��)��ԭ���Ĵ� |
| B����Һ��������������Һ��c(Ca2��)��c(SO42��)����С |
| C����Һ������CaSO4��������Һ��c(Ca2��)��c(SO42��)����С |
| D����Һ��������������������Һ��c(SO42��)��ԭ���Ĵ� |
��50 mL 0.018 mol��L-1 AgNO3��Һ�м���50 mL 0.02 mol��L��1���ᡣ��֪AgCl(s)���ܶȻ�����Ksp= 1��10��10����Ϻ���Һ������仯���Բ��ơ�����˵������ȷ����( )
| A����Ϻ���Һ�п϶��г������� |
| B���������ɺ���Һ��Ag+��Ũ��Ϊ10-5 mol��L-1 |
| C���������ɺ���Һ��pH��2 |
| D����Ϻ������¶ȣ���Һ��Ag+��Ũ������ |
��0.1 mol/L�Ĵ�������Һ�м����������������ʣ���Һ������Ũ�ȴ�С��ϵ��ȷ����( )
| A��ˮ��c(CH3COO-)��c(Na+)��c(OH-)��c(H+) |
| B��0.1 mol/L���c(Na+)=c(Cl-)��c(H+)��c(CH3COO-)��c(OH-) |
| C��0.1 mol/L���c(Na+)��c(CH3COO-)��c(H+)��c(OH-) |
| D��0.1 mol/L�������ƣ�c(Na+)��c(CH3COO-)��c(OH-)��c(H+) |
��������������У�����֤������������ʵ���( )
| A��1 mol / L������Һ��c(H+)="0.01" mol/L |
| B����������ˮ���κα������� |
| C��10 mL 1 mol/L����ǡ����10 mL 1 mol/L NaOH��Һ��ȫ��Ӧ |
| D��������Һ�ĵ����Ա�������� |
25 ��ʱ��Ũ�Ⱦ�Ϊ0.1 mol��L��1��HA��Һ��BOH��Һ��pH�ֱ���1��11������˵����ȷ����(����)
| A����0.1 mol��L��1BA��Һ�У�c(A��)��c(H��)��c(BOH)��c(B��) |
| B������0.1 mol��L��1 BOH��Һϡ����0.001 mol��L��1����Һ��pH��9 |
| C������һ��������������Һ��Ϻ�pH��7������Һ�У�c(A��)>c(B��) |
| D��������������Һ�������1��1��ϣ�����Һ�У�c(A��)>c(B��)>c(H��)>c(OH��) |