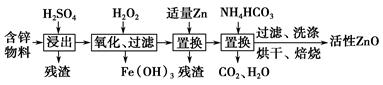
��Ŀ����
�������洦����Ƥ�����ơ�ӡȾ�ȶ�������ɸ���Ⱦ�����۸������۸����Ըߣ����ױ����������������������
�Ź�ҵ�ϴ������Ժ�Cr2O72����ˮ�ķ������£�
����Cr2O72�������Է�ˮ�м���FeSO4��Һ��ʹCr2O72��ȫ��ת��ΪCr3����д���÷�Ӧ�����ӷ���ʽ�� ��
�ڵ�����Һ��pH��ʹCr3����ȫ������ʵ���Ҵ��Բⶨ��ҺpH�ķ���Ϊ ��25�棬��������Һ��pH=8������Һ�в���Cr3�������ʵ���Ũ��Ϊ mol/L������֪25��ʱ��Ksp[Cr(OH)3]=6.3��10��31��
�Ƹ�Ԫ����Ũ�ȵIJⶨ��ȷ��ȡ25.00mL��Cr2O72����Cr3�������Է�ˮ�������м���������(NH4)2S2O8��Һ��Cr3��������Cr2O72������г�ȥ������(NH4)2S2O8����������Һ�м��������KI��Һ����ַ�Ӧ���Ե���Ϊָʾ���������еμ�0.015mol/L��Na2S2O3����Һ���յ�ʱ����Na2S2O3��Һ20.00mL��
�����ˮ�и�Ԫ����Ũ�ȣ���λ��mg��L��1��д��������̣���
��֪�ⶨ�����з����ķ�Ӧ���£�
��2Cr3����3S2O82����7H2O =Cr2O72����6SO42����14H��
��Cr2O72����6I����14H��=2Cr3����3I2��7H2O
��I2��2S2O32��=2I����S4O62��
��Cr2O72����6Fe2����14H��=2Cr3����6Fe3����7H2O
�ڽ�pH��ֽ���ڽྻ�ı������ϣ��ò�����պȡ��Һ�� ����pH��ֽ�ϣ��������ɫ������
6.3��10��13
���ɷ���ʽ��֪��Cr~3Na2S2O3
n(Na2S2O3)=20.00mL��0.015mol/L=3��10��4mol
n(Cr)=1��10��4mol
m(Cr)=1��10��4mol��52g��mol��1=5.2��10��3 g=5.2mg
��ˮ�и�Ԫ����Ũ��=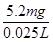 =208 mg��L��1
=208 mg��L��1
���������������1���ٷ���������ԭ��Ӧ���������ӱ�����Ϊ�����ӣ�Cr2O72����6Fe2����14H��=2Cr3����6Fe3����7H2O����ʵ���Ҵ��Բⶨ��ҺpH�ķ�����ʹ��pH��ֽ����pH��ֽ���ڽྻ�ı������ϣ��ò�����պȡ��Һ������pH��ֽ�ϣ��������ɫ�����գ�����Һ�в���Cr3�������ʵ���Ũ��Ϊ
Ksp[Cr(OH)3]��(OH��)3=6.3��10��31��10��18=6.3��10��13��
��2���ɷ���ʽȷ����ϵʽ��Cr~3Na2S2O3
n(Na2S2O3)=20.00mL��0.015mol/L=3��10��4mol
n(Cr)=1��10��4mol
m(Cr)=1��10��4mol��52g��mol��1=5.2��10��3 g=5.2mg
��ˮ�и�Ԫ����Ũ��=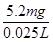 =208 mg��L��1
=208 mg��L��1
���㣺���鹤ҵԪ�غ����IJⶨ�ķ����������й����⡣

 ��ĩ���100�ִ��½����ȫ�Ծ�ϵ�д�
��ĩ���100�ִ��½����ȫ�Ծ�ϵ�д�����˵����ȷ���ǣ� ��
| A��Ħ�����߸�����������֮һ |
| B��1mol�� |
| C��Ħ���DZ�ʾ���ʵ�������λ |
| D��ÿĦ�����ʶ����а����ӵ�������ָ���� |
��֪ij��84����Һ��ƿ�岿�ֱ�ǩ��ͼ��ʾ���á�84����Һ��ͨ��ϡ��100��(���֮��)��ʹ�á���ش��������⣺
(1)�á�84����Һ�������ʵ���Ũ��ԼΪ mol��L��1��
(2)ijͬѧȡ100 mL�á�84����Һ����ϡ�ͺ�����������ϡ�ͺ����Һ��c(Na��)�� mol��L��1��
(3)��ͬѧ���ĸá�84����Һ�����䷽������NaClO��������480 mL��NaClO��������Ϊ25%������Һ������˵����ȷ���� (�����)��
| A����ͼ��ʾ�������У��������Dz���Ҫ�ģ�����Ҫһ�ֲ������� |
| B������ƿ������ˮϴ����Ӧ��ɺ����������Һ���� |
| C�����ƹ����У�δ������ˮϴ���ձ��Ͳ��������ܵ��½��ƫ�� |
| D����Ҫ����NaClO���������Ϊ143.0 g |

(4)��84����Һ����ϡ������ʹ�ÿ���ǿ����������ij����С����Ա��98%(�ܶ�Ϊ1.84 g��cm��3)��Ũ��������2 000 mL 2.3 mol��L��1��ϡ����������ǿ��84����Һ��������������
�������Ƶ�ϡ�����У�H�������ʵ���Ũ��Ϊ mol��L��1��
������Ũ��������Ϊ mL��
���ǻ��õļ����Ԫ�أ��Ƽ��仯�������������������й㷺��Ӧ�á�
������м��㣺
(1)��������(NaN3)��ײ����ȫ�ֽ�����ƺ͵������ʿ�Ӧ����������ȫ���ҡ�������40.32 L(��״����)������������Ҫ��������________g��
(2)���غϽ���ں˷�Ӧ���������Ƚ���Һ��5.05 g���غϽ�����200 mLˮ����0.075 mol������
�ټ�����Һ�����������ӵ����ʵ���Ũ��(������Һ����仯)��
_____________________________________________________________
�ڼ��㲢ȷ�������غϽ�Ļ�ѧʽ��
_____________________________________________________________
(3)����������Һ�����������ˣ��õ��������Ƶ���Һ�������Һ��ͨ�������̼�������з�Ӧ��
2NaAlO2��3H2O��CO2=2Al(OH)3����Na2CO3
��֪ͨ�������̼336 L(��״����)������24 mol Al(OH)3��15 mol Na2CO3����ͨ����Һ�Ķ�����̼Ϊ112 L(��״����)���������ɵ�Al(OH)3��Na2CO3�����ʵ���֮�ȡ�
_________________________________________________________________
(4)�����£���ȡ��ͬ����������Ʒ����ˮ���������к���pH��7��Ȼ����Һ���ɵ��Ȼ��ƾ��壬���ɹ����в�Ʒ����ʧ��
| | ������������(g) | �Ȼ�������(g) |
| �� | 2.40 | 3.51 |
| �� | 2.32 | 2.34 |
| �� | 3.48 | 3.51 |
����ʵ��٢ڢ������������ƾ��������ʣ���ʵ�����ݿɿ���ͨ�����㣬�����ͱȽ��ϱ�3�����ݣ��������ۡ�
 2O3��
2O3��