��Ŀ����
A��B��C��D��E��F�ܱ�ǰ�������еij���Ԫ�أ��������Ϣ���±���
| Ԫ�� | �����Ϣ |
| A | A�����ڱ���ԭ�Ӱ뾶��С��Ԫ�� |
| B | BԪ�ص�ԭ�Ӽ۵����Ų�Ϊns11np14 |
| C | M�Ļ�̬ԭ��L���������K���������3�� |
| D | D�ǵ��������е�һ��������С��Ԫ�� |
| E | E�ǵؿ��к������Ľ���Ԫ�� |
| F | �ж��ֻ��ϼۣ���ij�ָ������ӵļ۵��Ӿ��н��ȶ��İ�����ṹ |
��1��Fλ��Ԫ�����ڱ���λ�� �����̬ԭ�Ӻ���۵����Ų�ʽΪ ��
��2��B�ĵ縺�Ա�M�� �����С������B2A3������
 ����
����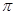 �������֮��Ϊ ��
�������֮��Ϊ ����3��д��E�ĵ�����D������������ˮ������Һ��Ӧ�Ļ�ѧ����ʽ�� ��
��4����֪ÿ5.4gE������ͼ�F�������ﷴӦ���ų�346.2kJ������������д���÷�Ӧ���Ȼ�ѧ����ʽ�� ��
(12�֣�ÿ��2��)
��1���������ڢ���3d64s2
��2��С 5��1
��3��2A1+2NaOH+6H2O=2Na[Al(OH)4]+3H2��
��4��2Al(s)+3FeO(s)=2A12O3(s)+3Fe(s) ��H=-3462kJ/mol
�����������������������������֪AΪ��Ԫ�أ�BΪ̼Ԫ�أ�CΪ��Ԫ�أ�DΪ��Ԫ�أ�EΪ��Ԫ�أ�FΪ��Ԫ�ء���2��Ԫ�صķǽ�����Խǿ����縺��Խ��C2H4��������1��˫����4������������1���м���5���Ҽ�����4��д����ѧ����ʽ���ɼ������H����д�Ȼ�ѧ����ʽʱӦע��д�������ʵ�״̬��
���㣺�������ʽṹ��Ԫ�������ɣ����鿼����Ԫ���ƶϺ����ʽṹ�����ʵ����ա�

��ϰ��ϵ�д�
 ���Ͱ�ͨ��ĩ���ϵ�д�
���Ͱ�ͨ��ĩ���ϵ�д�
�����Ŀ
�±���Ԫ�����ڱ���һ���֣�����Ҫ��ش��������⡣
| �� ���� | ��A | ��A | ��A | ��A | ��A | ��A | ��A | O |
| 2 | | | | E | H | F | I | |
| 3 | A | C | D | | | | G | R |
| 4 | B | | | | | | | |
��1��ʮ��Ԫ���л�ѧ��������õ�Ԫ����________����Ԫ�ط��ţ���
��2��A��C��D����Ԫ�ص��������Ӧ��ˮ������м�����ǿ����________���ѧʽ����
��3��IԪ�ظ�AԪ���γɻ�����ĵ���ʽ��________���������ոû�����ʱ�������________ɫ��
��4��G�ĵ��ʺ�B������������Ӧˮ���ﷴӦ�����ӷ���ʽ��__________________��
Ԫ��A��F���γ����ֻ����д�����н��ȶ��Ļ�������CO2��Ӧ���������Ļ�ѧ����ʽ ________________________��
�йض�����Ԫ��A��B��C��D��E��F����Ϣ���£�
| Ԫ�� | �й���Ϣ |
| A | ����������Ӧ��ˮ����ף���������̬�⻯��ң���Ӧ������ |
| B | �����������Ǵ�����������2�� |
| C | M������3������ |
| D | ������ԭ�Ӱ뾶��������Ԫ�� |
| E | �䵥���ǵ���ɫ���� |
| F | �������������۴�����Ϊ6 |
��ش��������⣺
��1��д��ʵ������ȡ�ҵĻ�ѧ����ʽ ��
��2������˵����ȷ���� (�����)��
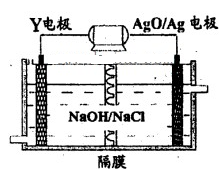
��ʵ���ҿ�����ͼ��ʾװ����ȡB�����������

����C�������ɵIJ۳����������������Ũ��Һ
�� C��ͭ��ϡ������ɵ�ԭ��أ�C�缫����ԭ
�� D������������ȼ�պ�IJ�������ڷ����������������
�ݹ����������������У�������̼����ǿ��ƺ�����BO2���������ЧӦ������Ч;��֮һ
 �� DF�ĵ���ʽΪH��Cl��
�� DF�ĵ���ʽΪH��Cl����3����E�ij������������������ʹƷ����Һ��ɫ��ͨ����CuSO4��NaCl��ϵ�Ũ��Һ�У���Һ��ɫ��dz��������ɫ������ȡ�ó�������Ԫ������������������֪���к�Cl��35.7%��Cu��64.3%�������������������Ӧ�е������� ��
A��Ư�� B�������� C����ԭ��
��4�����û�ѧ����������֤��3���е��������Ҫд��ʵ�鷽�����Լ���Ԥ�ڿɹ۲쵽������ ��