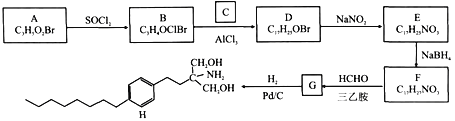
��Ŀ����
����Ŀ��N��Fe��������Ҫ��Ԫ�أ��䵥�ʼ�����������������ж��й㷺��Ӧ�á�
(1)��̬Nԭ������ܼ��ĵ���������ͼ��״��__________���������______�ֲ�ͬ�˶�״̬�ĵ��ӡ�
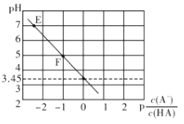
(2)��һ������N_____O�����������������������ԭ����_______________________��
(3)�ڸ�ѹ�µ����ᷢ���ۺϵõ��߾۵���������ÿ����ԭ��������������ԭ�ӽ���γɿռ���״�ṹ���߾۵��ľ���������__________����ԭ�ӵ��ӻ��������Ϊ__________��
(4)����������BN����ʯī����ṹ���ƣ���ԭ�Ӻ͵�ԭ�ӽ�����������ʯī���Ե��������BNȴ���ܵ��磬��ԭ����_____________________________��
(5)�����ᣨHN3��������������������ҪӦ�á������ᣨHN3������HNO2�����£�N2H4���Ƶã���ѧ����ʽ��N2H4 + HNO2��HN3 + 2H2O�����������������_________��
A��HN3��N2H4�����ɼ��Լ��ͷǼ��Լ����ɵķǼ��Է���
B��NaN3�ľ����ܴ���KN3�ľ�����
C��HN3�������ĸ�ԭ�ӿ�����һ��ֱ����
D�������ᣨHN3����ˮ���γɷ��Ӽ����
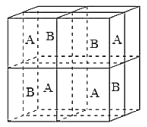
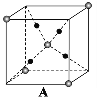
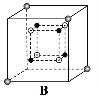
(6)ij�����������������ᄃ����ͼ��ʾ������A��B������ɡ���û�������Fe2����Fe3����O2���ĸ�������__________������������ȣ�����֪�þ���ľ�������Ϊa nm�������ӵ�������ֵΪNA����þ�����ܶ���_______ g![]() cm��3���ú�a��NA�Ĵ���ʽ��ʾ����
cm��3���ú�a��NA�Ĵ���ʽ��ʾ����
![]()
���𰸡��Ĵ��Σ��������Σ� 7 �� Nԭ�ӵ�2p���Ϊ���ȶ��İ�����ṹ����Oԭ��ʧȥһ�����Ӻ�2p�����Ϊ���ȶ��İ�����ṹ������Nԭ�ӵĵ�һ�����ܴ���Oԭ�� ԭ�Ӿ��� sp3 BN��Nԭ�ӵ縺�Դ�ʹNԭ��2p����ϵĵ��ӶԱ�������Nԭ���ϣ����������ƶ�����˲����� AC 1��2��4 ![]()
��������
��1��Nԭ�Ӻ˵����Ϊ7����̬N ԭ������ܼ�Ϊ2p�ܼ���
��2��Nԭ�ӵ�p���Ϊ������Ƚ��ȶ�����ԭ�ӱ���ԭ������ʧȥ���ӣ�
��3��������ÿ����ԭ��������������ԭ�ӽ���γɿռ���״�ṹ���ɴ��жϾ������ͣ�ÿ��Nԭ�Ӻ���һ���µ��Ӷԣ����ݼ۲���ӶԻ��������жϵ�ԭ�ӵ��ӻ�������ͣ�
��4����Ϊ���ĵ縺�Խϴ��ƽ�����ص�p���������������ϵĵ����ںܴ�̶��ϱ������ڵ�����Χ�����������ƶ���
��5��A��HN3��N2H4�м��м��Թ��ۼ���Ҳ�зǼ��Թ��ۼ���B��NaN3��KN3Ϊ�ṹ���Ƶ����Ӿ��壬Na+��K+�����ͬ��Na+�뾶С��K+���뾶ԽС��������Խ��C��HN3��N3-Ϊֱ�߽ṹ����H������NΪsp2�ӻ���D��HN3��Ҳ���ڵ縺�Խϴ��N��
��6��Fe2+���Ӵ��ھ����Ķ��㡢�����Լ�Aλ������������ġ�O2-λ��A��BС��������ڲ���ÿ��С�������ڲ�����4����Fe3+���Ӵ��ھ���Bλ��С�������ڲ�����̯�����㾧����Fe2+��Fe3+��O2-�ĸ��������㾧����������Ͼ�������=�����ܶ�������������㾧���ܶȡ�
��1������7��Ԫ�أ����ڵڶ�����VA�壬��������Ų�ʽΪ1s22s22p3������ܼ�Ϊ2p����������״Ϊ�����Σ�����7�������˶�״̬����ͬ������7�ֲ�ͬ�˶�״̬�ĵ��ӡ�
��2��Oԭ�ӵļ۵����Ų�Ϊ2s22p4��Nԭ�ӵļ۵����Ų�Ϊ2s22p3��p���Ϊ������Ƚ��ȶ�����ԭ�ӱ���ԭ������ʧȥ���ӣ��ʵ�Ԫ�صĵ�һ�����ܴ�����Ԫ�صģ�
��3��������ÿ����ԭ��������������ԭ�ӽ���γɿռ���״�ṹ����˾���Ϊԭ�Ӿ��壻ÿ��N�γ�3��N-N����������1�Թµ��Ӷԣ��ӻ������ĿΪ4��Nԭ���ӻ���ʽΪsp3��
��4����Ϊ���ĵ縺�Խϴ��ƽ�����ص�p���������������ϵĵ����ںܴ�̶��ϱ������ڵ�����Χ�����������ƶ�����������BN�����磻
��5��A��HN3��N2H4�м��м��Թ��ۼ���Ҳ�зǼ��Թ��ۼ�������ǰ���Ǽ��Է��ӣ������ǷǼ��Է��ӣ���A����B��NaN3��KN3Ϊ�ṹ���Ƶ����Ӿ��壬Na+��K+�����ͬ��Na+�뾶С��K+���뾶ԽС��������Խ����NaN3�ľ����ܴ���KN3�ľ����ܣ���B��ȷ��C��HN3��N3-Ϊֱ�߽ṹ����H������NΪsp2�ӻ��������ĸ�ԭ�Ӳ�������ͬһֱ���ϣ���C����D��HN3��Ҳ���ڵ縺�Խϴ��N����ˮ���γɷ��Ӽ��������D��ȷ���ʴ�ΪAC��
��6��Fe2+���Ӵ��ھ����Ķ��㡢�����Լ�Aλ������������ġ�O2-λ��A��BС��������ڲ���ÿ��С�������ڲ�����4����Fe3+���Ӵ��ھ���Bλ��С�������ڲ���������Fe2+������Ŀ=4+8��![]() +6��
+6��![]() =8��Fe3+������Ŀ=4��4=16��O2-������Ŀ=4��8=32����Fe2+��Fe3+��O2-�ĸ�����Ϊ8:16:32=1:2:4��Fe��Oԭ����Ŀ֮��=24:32=3:4���������ﻯѧʽΪFe3O4�������൱����8����Fe3O4������������=8��
=8��Fe3+������Ŀ=4��4=16��O2-������Ŀ=4��8=32����Fe2+��Fe3+��O2-�ĸ�����Ϊ8:16:32=1:2:4��Fe��Oԭ����Ŀ֮��=24:32=3:4���������ﻯѧʽΪFe3O4�������൱����8����Fe3O4������������=8��![]() g����������Ϊa nm����8��
g����������Ϊa nm����8��![]() g=��g
g=��g![]() cm��3����a��10-7 cm��3�������=
cm��3����a��10-7 cm��3�������=![]() g
g![]() cm��3��
cm��3��