��Ŀ����
����Ŀ����ˮ�DZ������Ȼ��Դ�����ú�ˮ���Եõ�һϵ�в�Ʒ��Ҳ���Խ��з���������
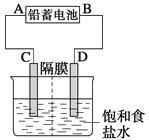
��1�������ȼҵ��Ʒ��������SO2��������������ͼ��ʾ��
�١�����װ�á��з�����Ӧ�����ӷ���ʽ��__________________________��
������������ѭ�����õ�������________��
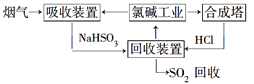
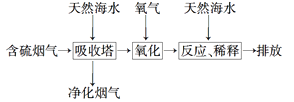
��2�����ú�ˮ���������Ч�ؽ��úȼ�չ������ŷŵ�SO2��ɵ�һϵ�л������⡣�乤��������ͼ��ʾ����Ȼ��ˮ���պ������������Ҫ���������������������䷴Ӧ�Ļ�ѧ����ʽ��_____________________��������ĺ�ˮ��Ҫ�����������ƣ���֮��Ϻ�����ŷš��ò�������ҪĿ����____________________��
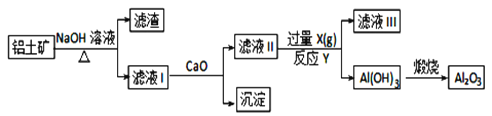
��3���Ӻ�ˮ���ᴿ���κ��ĸҺ�к���K����Na����Mg2���������ӡ���ĸҺ����һϵ�еļӹ����Ƶý���þ��
�ٴ����ӷ�Ӧ�ĽǶ�˼������ĸҺ�м���ʯ���������������___________��
��Ҫ����MgCl2��6H2O�Ƶ���ˮ�Ȼ�þ��Ӧ��ȡ�Ĵ�ʩ��________________��
�۵�����ڵ���ˮ�Ȼ�þ���õ�þ�������ض��Ļ�������ȴ��Ϊ����þ�����������п�������þ��������ȴ������________(����ĸ)��
A��Ar�� B��CO2 C������ D��O2 E��ˮ����
���𰸡�SO2��OH��=HSO3�� 2H2SO3��O2=2H2SO4 NaCl ʹ�������������ᷢ���кͷ�Ӧ ����Mg2��[����ȡMg(OH)2] ��HCl��������ˮ������MgCl2��ˮ�� A
��������
����(1) ���ȼҵ��Ӧ�Ļ�ѧ����ʽΪ��2NaCl+H2O![]() 2NaOH+H2��+Cl2��.��Һ����Ҫ�ɷ���NaOH������SO2������ͨ�����Һʱ������Ӧ��SO2��2NaOH=Na2SO3+H2O ��SO2����ʱ����SO2��NaOH=NaHSO3����Ӧ�����ӷ���ʽΪ��SO2��2OH��=SO32-+H2O��SO2��OH��=HSO3-����������ͼ���Կ�����������������ѭ�����õ�������NaCl����2�����������������������պ������������Ȼ��ˮ�ķ�Ӧԭ���Ļ�ѧ����ʽ��2H2SO3��O2=2H2SO4��������ĺ�ˮ�������ᣬˮ��Һ�����ԣ�������Ҫ�����������ƣ���֮��Ϻ�����ŷţ��ò�������ҪĿ����ʹ�������������ᷢ���кͷ�Ӧ����3������ĸҺ�м���ʯ���������������ʹMg2��ת��ΪMg(OH) 2������ȥ����MgCl2��ǿ�������Σ�����ʱ�λ������ڽᾧˮ�У��η���ˮ�ⷴӦ����Mg(OH) 2��HCl��HCl����ˮ�ֵ��������ӷ������õ�����Mg(OH) 2���塣Ԫ��Ҫ����MgCl2��6H2O�Ƶ���ˮ�Ȼ�þ��Ӧ��ȡ�Ĵ�ʩ����HCl��������ˮ��������MgCl2ˮ�⡣������Mg��Ժ�ǿ���ڸ���ʱ����������е�O2��ˮ����������Ӧ��Ҳ����CO2������Ӧ����MgO��C������Ҫ�ڶ�������Ar�Ļ�������ȴ��ѡ��ΪA��
2NaOH+H2��+Cl2��.��Һ����Ҫ�ɷ���NaOH������SO2������ͨ�����Һʱ������Ӧ��SO2��2NaOH=Na2SO3+H2O ��SO2����ʱ����SO2��NaOH=NaHSO3����Ӧ�����ӷ���ʽΪ��SO2��2OH��=SO32-+H2O��SO2��OH��=HSO3-����������ͼ���Կ�����������������ѭ�����õ�������NaCl����2�����������������������պ������������Ȼ��ˮ�ķ�Ӧԭ���Ļ�ѧ����ʽ��2H2SO3��O2=2H2SO4��������ĺ�ˮ�������ᣬˮ��Һ�����ԣ�������Ҫ�����������ƣ���֮��Ϻ�����ŷţ��ò�������ҪĿ����ʹ�������������ᷢ���кͷ�Ӧ����3������ĸҺ�м���ʯ���������������ʹMg2��ת��ΪMg(OH) 2������ȥ����MgCl2��ǿ�������Σ�����ʱ�λ������ڽᾧˮ�У��η���ˮ�ⷴӦ����Mg(OH) 2��HCl��HCl����ˮ�ֵ��������ӷ������õ�����Mg(OH) 2���塣Ԫ��Ҫ����MgCl2��6H2O�Ƶ���ˮ�Ȼ�þ��Ӧ��ȡ�Ĵ�ʩ����HCl��������ˮ��������MgCl2ˮ�⡣������Mg��Ժ�ǿ���ڸ���ʱ����������е�O2��ˮ����������Ӧ��Ҳ����CO2������Ӧ����MgO��C������Ҫ�ڶ�������Ar�Ļ�������ȴ��ѡ��ΪA��

 ��ʦָ����ĩ��̾�ϵ�д�
��ʦָ����ĩ��̾�ϵ�д�����Ŀ����������ƾ��壨Na2S2O3��5H2O��M=248 g��mol1����������Ӱ������ԭ�����ش��������⣺
��1����֪��Ksp(BaSO4)=1.1��1010��Ksp(BaS2O3)=4.1��105����������������г�������������ʣ�ѡ�������Լ����ʵ�鷽�����м��飺
�Լ���ϡ���ᡢϡH2SO4��BaCl2��Һ��Na2CO3��Һ��H2O2��Һ
ʵ�鲽�� | ���� |
��ȡ������Ʒ�������������ˮ | �ڹ�����ȫ�ܽ����ɫ������Һ |
��___________ | ��___________���д̼���������� |
�ݾ��ã�___________ | ��___________ |
��2������K2Cr2O7����Һ�����ⶨ��������ƵĴ��ȡ��ⶨ�������£�
����Һ���ƣ���ȡ1.2000 gij��������ƾ�����Ʒ��������в���ȴ������ˮ��__________���ܽ⣬��ȫ�ܽ��ȫ��ת����100 mL��_________�У�������ˮ��____________��
�ڵζ���ȡ0.00950 mol��L1��K2Cr2O7����Һ20.00 mL�������ữ��������KI��������Ӧ�� Cr2O72+6I+14H+![]() 3I2+2Cr3++7H2O��Ȼ���������������Ʒ��Һ�ζ���������ɫ��������Ӧ��I2+2S2O32
3I2+2Cr3++7H2O��Ȼ���������������Ʒ��Һ�ζ���������ɫ��������Ӧ��I2+2S2O32![]() S4O62+2I�����������Һ��Ϊָʾ���������ζ�������Һ__________����Ϊ�յ㡣ƽ�еζ�3�Σ���Ʒ��Һ��ƽ������Ϊ24.80 mL������Ʒ����Ϊ_________%������1λС������
S4O62+2I�����������Һ��Ϊָʾ���������ζ�������Һ__________����Ϊ�յ㡣ƽ�еζ�3�Σ���Ʒ��Һ��ƽ������Ϊ24.80 mL������Ʒ����Ϊ_________%������1λС������