��Ŀ����
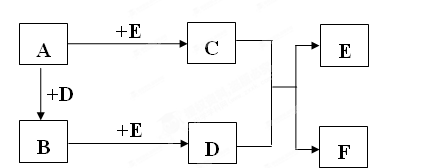
��20�֣�A��B��CΪ��ѧ�������ʣ�����һ��Ϊ������ͨ�������AΪ���塢BΪ����ɫ���塢CΪ��ɫ���塣D��E��F��G��H��X��Ϊ���������X����������ɫ���壬��ˮ��Һ��һ������ǿ����Һ��EΪ��ɫ���壬H�ڳ�����ΪҺ�塣����֮���ת����ϵ��ͼ��ʾ������ijЩ��Ӧ�����Ͳ��ַ�Ӧ��������ȥ����
��1��д���������ʵĻ�ѧʽ��D X ��
��2���ڷ�Ӧ��~���У�������������ԭ��Ӧ���� �����ţ���
��3����Ӧ�����ӷ���ʽΪ�� ��
��4����G��Һ�м���NaOH��Һ�۲쵽�������� ��
��5����Ӧ�ߵĻ�ѧ����ʽΪ ���÷�Ӧ��ÿ����0.3mol��A����ת�Ƶ��� mol��
��6����ȥD��Һ�л��е�����G�ķ����ǣ�
��7����D��Һ���Ʊ���ˮD�ķ����ǣ�
��8���ֱ�д��D��Һ��С�մ���Һ��Ӧ�����ӷ���ʽ�ǣ�

��1��д���������ʵĻ�ѧʽ��D X ��
��2���ڷ�Ӧ��~���У�������������ԭ��Ӧ���� �����ţ���
��3����Ӧ�����ӷ���ʽΪ�� ��
��4����G��Һ�м���NaOH��Һ�۲쵽�������� ��
��5����Ӧ�ߵĻ�ѧ����ʽΪ ���÷�Ӧ��ÿ����0.3mol��A����ת�Ƶ��� mol��
��6����ȥD��Һ�л��е�����G�ķ����ǣ�
��7����D��Һ���Ʊ���ˮD�ķ����ǣ�
��8���ֱ�д��D��Һ��С�մ���Һ��Ӧ�����ӷ���ʽ�ǣ�
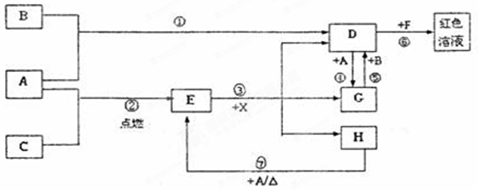
��1��FeCl3��1�֣� HCl��1�֣� ��2���ۢޣ�1�֣�
��3��Fe3++3SCN-=Fe(SCN)3����2�֣�
��4�����ɰ�ɫ������Ѹ�ٱ�Ϊ����ɫ�����ձ�Ϊ���ɫ����2�֣�
��5��3Fe+4H2O(g) Fe3O4+4H2 ��2�֣� 0.8��1�֣�
Fe3O4+4H2 ��2�֣� 0.8��1�֣�
��6������Һ��ͨ���������������������H2O2��
��7����HCl�����м�������FeCl3��Һ
��8��Fe3++ 3HCO3- = Fe(OH)3 ��+ 3CO2��
��3��Fe3++3SCN-=Fe(SCN)3����2�֣�
��4�����ɰ�ɫ������Ѹ�ٱ�Ϊ����ɫ�����ձ�Ϊ���ɫ����2�֣�
��5��3Fe+4H2O(g)
 Fe3O4+4H2 ��2�֣� 0.8��1�֣�
Fe3O4+4H2 ��2�֣� 0.8��1�֣���6������Һ��ͨ���������������������H2O2��
��7����HCl�����м�������FeCl3��Һ
��8��Fe3++ 3HCO3- = Fe(OH)3 ��+ 3CO2��
����������ͼ�⣬�ؼ�����ͻ�Ƶ㡣BΪ����ɫ���壬����B�����������ݷ�Ӧ�٢ܿ�֪��AӦ�����DZ�۵Ľ�����������D���Ȼ�����G���Ȼ�������X����������ɫ���壬��ˮ��Һ��һ������ǿ����Һ�����X���Ȼ��⡣����C��������E��������������H�ڳ�����ΪҺ�壬��H��ˮ���Ȼ�����F���ɺ�ɫ��Һ������F��KSCN��
��1����3���ԣ�2�������������������Լ��Ȼ�����KSCN��Һ�ķ�Ӧ������������ԭ��Ӧ��
��4���������������¶ȼ��ױ��������������������������������ɰ�ɫ������Ѹ�ٱ�Ϊ����ɫ�����ձ�Ϊ���ɫ��
��5������������ˮ������Ӧ��������������������������ʽΪ3Fe+4H2O(g) Fe3O4+4H2�����������������Ļ��ϼ��ǣ�8/3�ۣ�������Ҫ������8/3����ÿ����0.3mol��A����ת�Ƶ���0.8mol��
Fe3O4+4H2�����������������Ļ��ϼ��ǣ�8/3�ۣ�������Ҫ������8/3����ÿ����0.3mol��A����ת�Ƶ���0.8mol��
��6���Ȼ��������л�ԭ�ԣ����Գ�ȥ�Ȼ����е��Ȼ������ķ���������Һ��ͨ���������������������H2O2����
��7���Ȼ�������Һ�д���ˮ��ƽ�⣬���������������Ȼ��⣬����Ʊ��Ȼ������뷴֮ˮ�⣬������D��Һ���Ʊ���ˮD�ķ�������HCl�����м�������FeCl3��Һ��
��8��ԭ��̼������ˮ���Լ��ԣ������ܺ��Ȼ�����ٽ�������ʽΪFe3++ 3HCO3- = Fe(OH)3 ��+ 3CO2����
��1����3���ԣ�2�������������������Լ��Ȼ�����KSCN��Һ�ķ�Ӧ������������ԭ��Ӧ��
��4���������������¶ȼ��ױ��������������������������������ɰ�ɫ������Ѹ�ٱ�Ϊ����ɫ�����ձ�Ϊ���ɫ��
��5������������ˮ������Ӧ��������������������������ʽΪ3Fe+4H2O(g)
 Fe3O4+4H2�����������������Ļ��ϼ��ǣ�8/3�ۣ�������Ҫ������8/3����ÿ����0.3mol��A����ת�Ƶ���0.8mol��
Fe3O4+4H2�����������������Ļ��ϼ��ǣ�8/3�ۣ�������Ҫ������8/3����ÿ����0.3mol��A����ת�Ƶ���0.8mol����6���Ȼ��������л�ԭ�ԣ����Գ�ȥ�Ȼ����е��Ȼ������ķ���������Һ��ͨ���������������������H2O2����
��7���Ȼ�������Һ�д���ˮ��ƽ�⣬���������������Ȼ��⣬����Ʊ��Ȼ������뷴֮ˮ�⣬������D��Һ���Ʊ���ˮD�ķ�������HCl�����м�������FeCl3��Һ��
��8��ԭ��̼������ˮ���Լ��ԣ������ܺ��Ȼ�����ٽ�������ʽΪFe3++ 3HCO3- = Fe(OH)3 ��+ 3CO2����

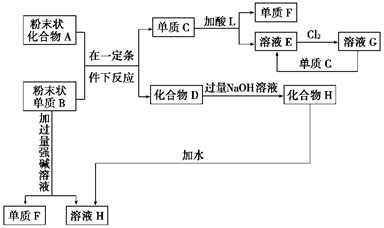
��ϰ��ϵ�д�
 ��˼ά������ҵ���ټ��ִ�ѧ������ϵ�д�
��˼ά������ҵ���ټ��ִ�ѧ������ϵ�д�
�����Ŀ


 ��
�� ��
�� �ף���ײ�������
�ף���ײ�������