��Ŀ����
9��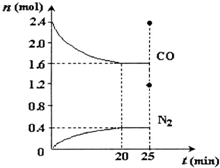 �Ժ������ʵ��о����������ż�Ϊ��Ҫ�����壮
�Ժ������ʵ��о����������ż�Ϊ��Ҫ�����壮��1��N2��O2��H2�֮����Է������Ϸ�Ӧ����֪��Ӧ���Ȼ�ѧ����ʽ���£�
N2��g��+O2��g��=2NO��g����H=+180.5kJ•mol-1��
2H2��g��+O2��g��=2H2O��g����H=-483.6kJ•mol-1��
N2��g��+3H2��g��=2NH3��g����H=-92.4kJ•mol-1��
�Ĵ�������Ӧ���Ȼ�ѧ����ʽΪ4NH3��g��+5O2��g��=4NO��g��+6H2O��g����H=-905kJ/mol��
��2������β��������һ����Ӧԭ��Ϊ��2NO��g��+2CO��g��?N2��g��+2CO2��g����һ���¶��£���2.8mol NO��2.4mol COͨ��̶��ݻ�Ϊ2L���ܱ������У���Ӧ�����в������ʵ����ʵ����仯��ͼ��ʾ��NO��ƽ��ת����Ϊ28.57%��0��20minƽ����Ӧ����v��NO��Ϊ0.02mol/��L•min����
���� ��1����֪����N2��g��+O2��g��=2NO��g����H=+180.5kJ•mol-1��
��2H2��g��+O2��g��=2H2O��g����H=-483.6kJ•mol-1��
��N2��g��+3H2��g��=2NH3��g����H=-92.4kJ•mol-1��
���ݸ�˹���ɣ��ڡ�3+�١�2-�ۡ�2�ɵã�4NH3��g��+5O2��g��=4NO��g��+6H2O��g����
��2����ͼ��֪��20min����ƽ�⣬ƽ��ʱ���ɵ���Ϊ0.4mol���ɷ���ʽ��֪����NOΪ0.4mol��2=0.8mol��ת����=$\frac{���ʵ����仯��}{��ʼ���ʵ���}$��100%������v=$\frac{��c}{��t}$����v��NO����
��� �⣺��1����֪����N2��g��+O2��g��=2NO��g����H=+180.5kJ•mol-1��
��2H2��g��+O2��g��=2H2O��g����H=-483.6kJ•mol-1��
��N2��g��+3H2��g��=2NH3��g����H=-92.4kJ•mol-1��
���ݸ�˹���ɣ��ڡ�3+�١�2-�ۡ�2�ɵã�4NH3��g��+5O2��g��=4NO��g��+6H2O��g����H=-905kJ/mol��
�ʴ�Ϊ��4NH3��g��+5O2��g��=4NO��g��+6H2O��g����H=-905kJ/mol��
��2����ͼ��֪��20min����ƽ�⣬ƽ��ʱ���ɵ���Ϊ0.4mol���ɷ���ʽ��֪����NOΪ0.4mol��2=0.8mol��NOת����=$\frac{0.8mol}{2.8mol}$��100%=28.57%��v��NO��=$\frac{\frac{0.8mol}{2L}}{20min}$=0.02mol/��L•min����
�ʴ�Ϊ��28.57%��0.02mol/��L•min����
���� ���⿼�黯ѧƽ����㡢��˹����Ӧ�ã��ѶȲ���ע��Ի���֪ʶ���������գ�

 ��У����ϵ�д�
��У����ϵ�д�| A�� | �ס������ǻ�ѧ��ת��Ϊ���ܣ������ǵ���ת��Ϊ��ѧ�� | |
| B�� | C1��C2�ֱ���������������пƬ����Ƭ�϶�����������Ӧ | |
| C�� | C1��C3�ų���������ͬ��ͭƬ����Ƭ�ų�������Ҳ��ͬ | |
| D�� | �ס�������Һ��pH�����ߣ�������Һ��pH��С |
| A�� | 2.4 g����þ����������ĿΪ0.2 NA | |
| B�� | ��״����22.4L CH4����ԭ����ĿΪNA | |
| C�� | 17 g NH3������������ĿΪ10 NA | |
| D�� | ���ʵ���Ũ��Ϊ1mol/L��MgCl2��Һ�У�����Cl-����Ϊ2NA |
���¶�T1��T2ʱ���ֱ�0.50molCH4��1.2molNO2�������Ϊ1L���ܱ������У����n��CH4����ʱ��仯�������±���
| �¶� | ʱ��/min n/mol | 0 | 10 | 20 | 40 | 50 |
| T1 | n��CH4�� | 0.50 | 0.35 | 0.25 | 0.10 | 0.10 |
| T2 | n��CH4�� | 0.50 | 0.30 | 0.18 | �� | 0.15 |
| A�� | 10 min�ڣ�T1ʱ�ԣ�CO2����T2ʱС | B�� | �¶ȣ�T1��T2 | ||
| C�� | a��0 | D�� | ƽ�ⳣ����K��T1����K��T2�� |
| A�� | 0.5 mol CO+2 mol H2O��g��+1 mol CO2+1 mol H2 | |
| B�� | 1 mol CO+1 mol H2O��g��+1 mol CO2+1 mol H2 | |
| C�� | 0.5 mol CO+1.5 mol H2O��g��+0.4 mol CO2+0.4 mol H2 | |
| D�� | 0.5 mol CO+1.5 mol H2O��g��+0.5 mol CO2+0.5 mol H2 |
| A�� | AlCl3��Һ�м������ϡ��ˮ��Al3++4NH3•H2O�TAlO${\;}_{2}^{-}$+4NH${\;}_{4}^{+}$+2H2O | |
| B�� | �����ڹ���ϡ�����У�Fe+4H++NO3-�TFe3++NO��+2H2O | |
| C�� | ��Ba��OH��2��Һ�еμ�ϡ���Ba2++OH-+H++SO${\;}_{4}^{2-}$�TBaSO4��+H2O | |
| D�� | ������Һ��NaHCO3��Һ��Ӧ��H++HCO3-�TCO2��+H2O |
| A�� | ���ڿ�����ȼ��ʱ���ۻ�����ȼ�գ�������õIJ�����Na2O2 | |
| B�� | �����ڿ������γ���һ�������ܵ�����Ĥ�������ڲ�����������������Ᵽ�� | |
| C�� | �ơ���������������Եķǽ���������������Ӧ��������Ӧ�����κ����� | |
| D�� | �����ڳ�ʪ�Ŀ������γɵ����������ɣ����ܱ����ڲ���� |
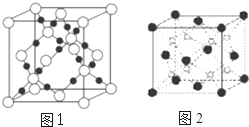 ���ֶ�����Ԫ��A��B��C��D��ԭ���������ε���������A��B��C����Ԫ�ػ�̬ԭ�ӵ�2p�ܼ��϶���δ�ɶԵ��ӣ���δ�ɶԵ��Ӹ����ֱ���2��3��2��D��C �����γ�D2C��D2C2���ֻ�����ش��������⣺
���ֶ�����Ԫ��A��B��C��D��ԭ���������ε���������A��B��C����Ԫ�ػ�̬ԭ�ӵ�2p�ܼ��϶���δ�ɶԵ��ӣ���δ�ɶԵ��Ӹ����ֱ���2��3��2��D��C �����γ�D2C��D2C2���ֻ�����ش��������⣺