��Ŀ����
����Ŀ����ͼ��C��D��E��F��X��Y���Ƕ��Ե缫������Դ��ͨ�����ң��е����̪��Һ����F�������Ժ�ɫ��������˵����ȷ���ǣ�������
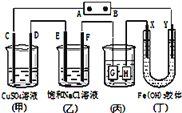
A. �������顢����ȼ�ϵ������Դ�������ΪKOH��Һ����A���ĵ缫��ӦʽΪ��C2H6 -14e-+ 18OH- = 2CO32- + 12H2O
B. ���ã�����װ�ø�ͭ������HӦ����Ag�����Һѡ��AgNO3��Һ
C. ������װ����Y���������ɫ���˵��������������������
D. C��D��
E. F�缫���е������ɣ��������ʵ�����Ϊ1:2:2:2
���𰸡�D
��������C��D��E��F��X��Y���Ƕ��Ե缫������Դ��ͨ�����ң��е����̪��Һ����F�������Ժ�ɫ����F������������C��E��G��X����������D��F��H��Y����������A��������B�Ǹ�����A��ȼ�ϵ���У��������������õ��ӷ�����ԭ��Ӧ���缫��ӦΪO2+4e-+2H2O=4OH-��ѡ��A����B�����ʱ���Ʋ����������Ƽ��������������ã�����װ�ø�ͭ������HӦ����Cu��G���������ҺѡAgNO3��Һ��ѡ��B����C��������ɵĽ����������ƶ���������װ����Y���������ɫ���˵�����������������Ӵ�����ɣ����岻���磬ѡ��C����D��C��D��E��F�缫��Ӧʽ�ֱ�Ϊ4OH--4e-=O2��+2H2O��Cu2++2e-=Cu��2Cl--2e-=Cl2����2H++2e-=H2������ת�Ƶ������ʱ�����ɵ��ʵ����ʵ���֮��Ϊ1��2��2��2��ѡ��D��ȷ����ѡD��

 ��˼ά������ҵ���ټ��ִ�ѧ������ϵ�д�
��˼ά������ҵ���ټ��ִ�ѧ������ϵ�д�����Ŀ������ʵ�鷽���ܴﵽʵ��Ŀ�ĵ����� ��
��� | A | B | C | D |
ʵ�鷽�� |
|
|
|
|
ʵ��Ŀ�� | ʵ�����Ʊ��������� | ���������ˮ | ��֤�����������������Ҵ���Һ�з�����ȥ��Ӧ��������ϩ | �ռ���ϩ����֤������ˮ�����ӳɷ�Ӧ |
A. A B. B C. C D. D
����Ŀ����֪���Ҷ��ᣨHOOC-COOH,�ɼ�дΪH2C2O4)�׳Ʋ���,1570Cʱ��ʼ�ֽ⣺
(1)̽�����������
250C H2C2O4 K1 = 5.4 x 10-2,K2 = 5. 4 x 10 -5 ��H2CO3 K1=4.5x10-7 K2= 4.7X10-11
���л�ѧ����ʽ������ȷ����________
A. H2C2O4 +CO32��=HCO3- +HC2O4�� B. HC2O4�� +CO32��= HCO3��+C2O42��
C. 2C2O42��+CO2+H2O=2HC2O4��+CO32�� D. H2C2O4 +CO32��=C2O42��+H2O+CO2
(2)̽������ֽ����
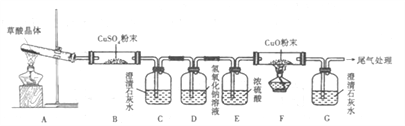
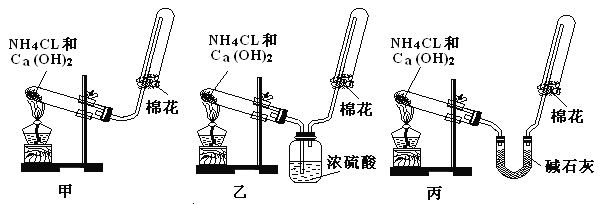
��ʵ���й۲쵽B��CuSO4��ĩ����,C�г���ʯ��ˮ�����,D������:_______��֤����CO�������ɵ������ǣ�_____________________
��д��H2C2O4�ֽ�Ļ�ѧ����ʽ_____________________
(3)̽�������Ի�ѧ��Ӧ���ʵ�Ӱ��
�ڼס�����֧�Թ��и�����4mLO.O1mol/T. KMnO4������Һ��2mL O.1mol/L H2C2O4��Һ�� �������Թ��м���һ���ƶ����MnSO4���壬ҡ�ȡ���д�±���
��Ӧ���� | ______________ |
ʵ����� | ______________ |
�Թ��з��ͷ�Ӧ�����ӷ���ʽ | ______________ |
(4)������KMnO4��Һ�ζ�Na2C2O4����Na2C2O4�Ĵ���
ʵ�鲽��:ȷ��ȡ2.OgNa2C2O4����,���1OO mL��Һ,ȡ��20.OOmL����ƿ��=����ƿ �м�������ϡH2SO4 ,��0.0160mol/L���Ը��������Һ�ζ����ζ����յ�ʱ���ĸ��������Һ25.OOmL0
�ٸ��������ҺӦװ��_______�ζ����С��������� ʽ�������� ʽ����
�ڵζ����յ�ʱ��ʵ�������ǣ�______________��
��Na2C2O4�Ĵ�����:______________
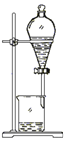
����Ŀ�����������գ�ij��ѧС�����������װ�ã��гֺͼ�������������ȥ������ⱥ��ʳ��ˮ�����õ������� H2 ��ԭ CuO ��ĩ���ⶨ Cu �����ԭ��������ͬʱ��֤�����������ԡ�

���������գ�
��1�� д����װ���з�Ӧ�Ļ�ѧ����ʽ______________________________��
��2�� Ϊ�������ʵ�飬��ȷ������˳��Ϊ A ��______,B��_____����д�ӿ���ĸ����
��3����װ���� a �Լ�������_______________��
��4�� �ⶨ Cu �����ԭ����������w g CuO ����Ӳ�ʲ������У�����������������õ����ݼ��� Cu �����ԭ������
���� 1 | ���� 2 | |
U ��+���� | Ӳ�ʲ�����+���� | |
��Ӧǰ��������/g | a | c |
��Ӧ���������/g | b | d |
����Ϊ�ϼѷ�����_____________����һ�������õ��� Cu �����ԭ��������_______��ѡ�ƫ�͡�����ƫ�ߡ���