��Ŀ����
����Ŀ����������Ҫ�Ļ���ԭ�ϣ�Ҳ�ǻ�ѧʵ������ر�����Ҫ�Լ������˾������ͨ�����⣬������һЩ��������ʡ�
��.þ��ϡ����ķ�ӦΪ��4Mg��10HNO3(ϡ)=4Mg(NO3)2��NH4NO3��3H2O
��1���á�˫���ŷ�����ʾ�÷�Ӧ�е���ת�Ƶķ������Ŀ___��
��2���÷�Ӧ���������뻹ԭ�������ʵ���֮��Ϊ___��
��3����ԭ������__��
��4������Ӧ������ת�Ƶĵ�����Ϊ1.505��1023����μӷ�Ӧ��Mg������Ϊ__��
��.ijһ��Ӧ��ϵ���з�Ӧ��������ﹲ5�����ʣ�S��H2S��HNO3��NO��H2O����֪ˮ�Ƿ�Ӧ�IJ���֮һ��д����Ӧ�Ļ�ѧ����ʽ__(������ƽ)��
���𰸡�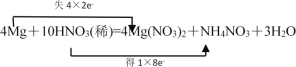 1��4 NH4NO3 3g H2S��HNO3��S����NO��H2O
1��4 NH4NO3 3g H2S��HNO3��S����NO��H2O
��������
��.��1���ɻ�ѧ����ʽ��֪����Ӧ��MgԪ�صĻ��ϼ���0�仯Ϊ+2��NԪ�صĻ��ϼ���+5��Ϊ+2��HNO3Ϊ��������NOΪ��ԭ���MgΪ��ԭ����Mg��NO3��2Ϊ���������Ӧ��ת�Ƶ�����ĿΪ6e��������˫���ŷ�����ʾ�÷�Ӧ�е���ת�Ƶķ������Ŀ ���ʴ�Ϊ��
���ʴ�Ϊ�� ��
��
��2����Ӧ��HNO3Ϊ������,10molHNO3�μӷ�Ӧֻ��1molHNO3����������MgΪ��ԭ��,���ݵ�ʧ������Ŀ�غ��֪�÷�Ӧ���������뻹ԭ�������ʵ���֮��Ϊ1��4���ʴ�Ϊ��1:4��
��3��������ԭ��Ӧ�У����������ϼ۽������ɻ�ԭ�����ԭ������NH4NO3���ʴ�Ϊ��NH4NO3��
��4������Ӧ������ת�Ƶĵ�����Ϊ1.505��1023����1.505��1023��6.02��1023mol-1=0.25mol����μӷ�Ӧ��Mg������Ϊ0.25mol![]() =3g���ʴ�Ϊ��3g��
=3g���ʴ�Ϊ��3g��
��.H2SΪ�����Ļ�ԭ����HNO3Ϊ���� ������������ԭ����ΪNO��ˮ�Ƿ�Ӧ�IJ���֮һ����Ӧ�Ļ�ѧ����ʽH2S��HNO3��S����NO��H2O(ĩ��ƽ)���ʴ�Ϊ��H2S��HNO3��S����NO��H2O(ĩ��ƽ)��

 ����ʦ��Сһ����ʦ������ҵϵ�д�
����ʦ��Сһ����ʦ������ҵϵ�д�