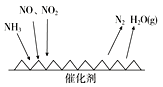
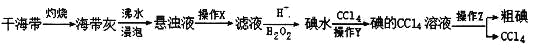��Ŀ����
����Ŀ��ij���᳦��ҩ����Ч�ɷֵĺϳ�·�����£����ַ�Ӧ��ȥ�Լ�����������

����������Ϣ�ش��������⣺
��1��B�Ľṹ��ʽ��____________________��
��2���ٵķ�Ӧ������________________;C��D�ķ�Ӧ������_____________��
��3�����жԿ��᳦��ҩ����Ч�ɷֿ��ܾ��е������Ʋ���ȷ����__________��
A.ˮ���Աȱ��Ӻã��ܶȱȱ��ӵĴ�
B.�ܷ�����ȥ��Ӧ�;ۺϷ�Ӧ
C.�ܺ��������Ʒ�Ӧ��1mol�÷��ӿ����к�3molNaOH
D.�������������
��4��E������NaOH��Һ��Ӧ�Ļ�ѧ����ʽ��_____________________________________.
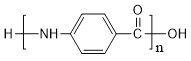
��5����������������E��ͬ���칹����_______�֣����к˴Ź�������������壬�����֮��1��2��2��2��1���칹��Ľṹ��ʽΪ_________________��
a����E������ͬ�Ĺ����� b�������ϵ�һ����ȡ������������
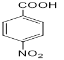
��6����֪![]() �ױ��������������������ʱ������һ��ȡ��������ȡ����������ڶ�λ�����������������Ȼ�ʱ��ȡ���ڼ�λ���ݴ������AΪԭ���Ʊ��߷��ӻ�����
�ױ��������������������ʱ������һ��ȡ��������ȡ����������ڶ�λ�����������������Ȼ�ʱ��ȡ���ڼ�λ���ݴ������AΪԭ���Ʊ��߷��ӻ����� ![]() �ĺϳ�·��_________________________________�������Լ���ѡ��
�ĺϳ�·��_________________________________�������Լ���ѡ��
���𰸡�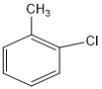 Fe��FeCl3 ȡ����Ӧ AD
Fe��FeCl3 ȡ����Ӧ AD 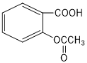 +3NaOH��
+3NaOH��![]() +CH3COONa+2H2O 4
+CH3COONa+2H2O 4 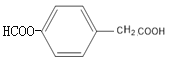 ��
�� ��
�� 
![]()
![]()
![]()

![]()

![]()
��������
C��C7H8O��C�� (CH3CO)2O��Ӧ����D��D����Ϊ �����
�����![]() ���ƣ���֪C��
���ƣ���֪C��![]() ��D��
��D�� ����A��
����A��![]() ��
��![]() ��һ�������·���ȡ����Ӧ����
��һ�������·���ȡ����Ӧ����![]() ��B��
��B��![]() ��
��![]() ˮ��Ϊ
ˮ��Ϊ![]() ��
�� ˮ������F��Fspan>��
ˮ������F��Fspan>��![]() ��G
��G![]()
![]() ����G��
����G��![]() ��
��
(1)�������Ϸ������ױ�����ȡ����Ӧ����B��B�Ľṹ��ʽ��![]() ��
��
(2)���Ǽױ������ϵ���ԭ�ӱ���ԭ��ȡ����Ӧ����Ӧ��������Fe��FeCl3��������![]() ��
�� ���ǻ��ϵ���ԭ�ӱ�-COCH3���棬��Ӧ������ȡ����Ӧ��
���ǻ��ϵ���ԭ�ӱ�-COCH3���棬��Ӧ������ȡ����Ӧ��
(3)A. ![]() �����Ȼ����Ȼ�����ˮ��������
�����Ȼ����Ȼ�����ˮ��������![]() ˮ���Աȱ��Ӻã���Է����������ܶȱȱ��ӵĴ�A��ȷ��
ˮ���Աȱ��Ӻã���Է����������ܶȱȱ��ӵĴ�A��ȷ��
B. ![]() ������ܷ�����ȥ��Ӧ����B����
������ܷ�����ȥ��Ӧ����B����
C. ![]() ���з��ǻ����Ȼ��������ܺ��������Ʒ�Ӧ��1mol�÷��ӿ����к�2molNaOH����C����
���з��ǻ����Ȼ��������ܺ��������Ʒ�Ӧ��1mol�÷��ӿ����к�2molNaOH����C����
D. ![]() �����Ȼ��Ͱ��������Լ����������м��ԣ���D��ȷ��
�����Ȼ��Ͱ��������Լ����������м��ԣ���D��ȷ��
(4) �����Ȼ���������������NaOH��Һ��Ӧ�Ļ�ѧ����ʽ��
�����Ȼ���������������NaOH��Һ��Ӧ�Ļ�ѧ����ʽ�� +3NaOH��
+3NaOH��![]() +CH3COONa+2H2O��
+CH3COONa+2H2O��
(5)a����E������ͬ�Ĺ����ţ�˵�������Ȼ��������� b�������ϵ�һ����ȡ�����������֣�˵���ṹ�Գƣ�����������E��ͬ���칹����![]() ��
�� ��
��![]() ��
��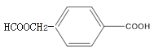 ��4�֣����к˴Ź�������������塢�����֮��1��2��2��2��1���칹��Ľṹ��ʽΪ��
��4�֣����к˴Ź�������������塢�����֮��1��2��2��2��1���칹��Ľṹ��ʽΪ�� ��
�� ��
��
(6)�ױ�����������Ӧ���ɶ������ױ����������ױ�����Ϊ�����������ᣬ�����������ỹԭΪ���������ᣬ���������ᷢ�����۷�Ӧ����![]() ���ϳ�·��Ϊ
���ϳ�·��Ϊ
![]()
![]()
![]()
![]()

![]()
![]() ��
��

����Ŀ�������й����ʼ����ʵ�飬������ȷ����(����)
ʵ����� | ���� | ʵ����� | |
A | ��ij��Һ�м��������ữ���Ȼ�����Һ | ���ɰ�ɫ���� | ��Һ��һ������ |
B | ��ij����ͨ��Ʒ����Һ�� | Ʒ����Һ��ɫ | ������һ����SO2 |
C | ��ij��Һ�м���KSCN��Һ | ��Ѫ��ɫ | ��Һ��һ������Fe3�� |
D | ��ij��Һ�м������� | ������ɫ���� | ��Һ��һ������ |
A.AB.BC.CD.D