��Ŀ����
����Ŀ�����Ͻ�����Ҫ����;�������������ȡ������������:
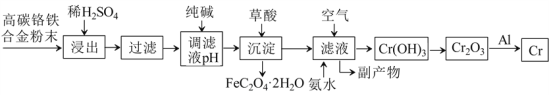
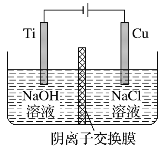
��֪: �� Cr+H2SO4=CrSO4+H2����
�� �����и�Ԫ�ؽ���֮��������Cr(OH)3֮����������ƶ�����״̬��������Һ�С�
��ش���������:
��1��ϡ������������У���������������Ĵ�ʩ�У�________________________ (дһ������) ��
��2���ô��������ҺpH���õ�ij������������������������ܵ��µĺ����_��_____________��
��3�������е��������������������������Ա����ж��ⶾ�������ʵĻ�ѧʽ��___________�����������ʵ����ʵĻ�ѧʽ��_____________��
��4���������ʵ�ֳ���ת����Ӧ��ѧ����ʽΪ��_______________________________________��
��5���������������ȷ�Ӧұ�����Ļ�ѧ����ʽΪ��_____________________________________��
��6������������Һ����Cr(OH)3�Ļ�ѧ����ʽΪ��_____________________________________��
��7������֪��Ӧ��֮�⣬�����������漰����Ҫ������ԭ��Ӧ��_____�����ֽⷴӦ��____����
���𰸡����ȡ����衢�ʵ����ϡ����Ũ�ȵ�(�𰸺�������) H2C2O4������������ʹCr2+ת���ɳ�������ģ� Na2SO4 (NH4)2SO4 Fe(OH)2+H2C2O4=FeC2O4��2H2O Cr2O3+2Al![]() Al2O3+2Cr 4CrSO4+O2+8NH3��H2O+2H2O=4Cr(OH)3��+4(NH4)2SO4 3 1
Al2O3+2Cr 4CrSO4+O2+8NH3��H2O+2H2O=4Cr(OH)3��+4(NH4)2SO4 3 1
��������
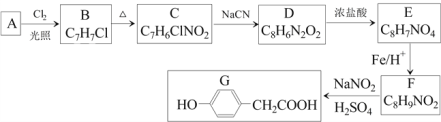
��̼�����Ͻ��ĩ����ϡH2SO4��ȡ������Cr��Fe�ܽ�����CrSO4��FeSO4�����˺��ô������pHʹFe2+ת����Fe��OH��2�������ټ�����ὫFe��OH��2ת��ΪFeC2O4��2H2O��������ȥ����ʱ��Һ����Ҫ��CrSO4��Na2SO4������Һ��ͨ����������백ˮ����Һ��Cr2+ת����Cr��OH��3�����������ķ�ӦΪ4CrSO4+O2+8NH3��H2O+2H2O��4Cr��OH��3��+4��NH4��2SO4���õ��ĸ�������Ҫ����NH4��2SO4��Na2SO4��Cr��OH��3���ȷֽ��Cr2O3��Cr2O3��Al�������ȷ�Ӧ����Cr��Al2O3���ݴ˷�������
��1��������������Է�Ӧ���ʵ�Ӱ���֪ϡH2SO4��������У���ߡ������ʡ��Ĵ�ʩ�У����ȡ����衢�ʵ����ϡH2SO4��Ũ�ȵȡ�
��2���ô��������ҺpH��ʹFe2+ת����Fe��OH��2�������ټ�����ὫFe��OH��2ת��ΪFeC2O4��2H2O��������ȥ����������������ܵ��µĺ���ǣ�һ����ʹCr2+ת���ɳ����������ģ�ʹCr�IJ��ʽ��ͣ���һ����ʹ����������H2C2O4������������
��3����ȥFe2+�����Һ����Ҫ����ΪCrSO4��Na2SO4������Һ��ͨ����������백ˮ����Һ��Cr2+ת����Cr��OH��3�������õ��ĸ�����Ϊ��NH4��2SO4��Na2SO4�����п����������Ա����ж��ⶾ�������ʵĻ�ѧʽ��Na2SO4���ⶾ��ԭ��ΪSO42-+Ba2+=BaSO4�������������ʵ����ʵĻ�ѧʽ�ǣ�NH4��2SO4����NH4��2SO4��һ�ֵ��ʡ�
��4��������ὫFe��OH��2����ת��ΪFeC2O4��2H2O������ת����Ӧ�Ļ�ѧ����ʽΪFe��OH��2+H2C2O4��FeC2O4��2H2O��
��5��Al��Cr2O3�������ȷ�Ӧ����Al2O3��Cr����Ӧ�Ļ�ѧ����ʽΪ2Al+Cr2O3![]() Al2O3+2Cr������Һ��ͨ����������백ˮCrSO4ת��ΪCr��OH��3������1molCr2+ʧȥ1mol��������1molCr��OH��3��1molO2�õ�4mol���ӣ����ݵ�ʧ�����غ㡢ԭ���غ㣬����Һ����Cr��OH��3�Ļ�ѧ����ʽΪ4CrSO4+O2+8NH3��H2O+2H2O��4Cr��OH��3��+4��NH4��2SO4��
Al2O3+2Cr������Һ��ͨ����������백ˮCrSO4ת��ΪCr��OH��3������1molCr2+ʧȥ1mol��������1molCr��OH��3��1molO2�õ�4mol���ӣ����ݵ�ʧ�����غ㡢ԭ���غ㣬����Һ����Cr��OH��3�Ļ�ѧ����ʽΪ4CrSO4+O2+8NH3��H2O+2H2O��4Cr��OH��3��+4��NH4��2SO4��
��6������Ӧ��֮�⣬�����������漰����Ҫ������ԭ��Ӧ���У�Fe+H2SO4��FeSO4+H2����4CrSO![]() Al2O3+2Cr����������ԭ��Ӧ��3�����ֽⷴӦΪ2Cr��OH��3
Al2O3+2Cr����������ԭ��Ӧ��3�����ֽⷴӦΪ2Cr��OH��3![]() Cr2O3+3H2O�����ֽⷴӦ��1����
Cr2O3+3H2O�����ֽⷴӦ��1����

����Ŀ���±������Ƕ�Ӧ���ʵ��۵㣺
��� | �� | �� | �� | �� | �� | �� | �� | �� |
���� | Na2O | NaCl | AlF3 | AlCl3 | BCl3 | Al2O3 | CO2 | SiO2 |
�۵�� | 920 | 801 | 1291 | 160 | -107 | 2072 | -57 | 1723 |
��1�������漰ԭ��������÷ǽ���ԭ�Ӻ�������Ų�ʽ��________________��ij�����ӵĹ����ʾʽΪ![]() ����������ռ�еĹ��������_____������______��������ͬ�ĵ��ӣ���_____�ֲ�ͬ�˶�״̬�ĵ��ӡ�
����������ռ�еĹ��������_____������______��������ͬ�ĵ��ӣ���_____�ֲ�ͬ�˶�״̬�ĵ��ӡ�
��2�����ʢٵĵ���ʽ��____________���ߵĽṹʽ��_______________��
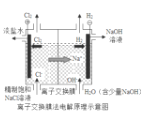
��3��������ˮ��Һ�����ԣ������ӷ���ʽ��ʾ��ԭ��_______________________________����������Һ�������ɲ����գ��õ��Ĺ�����_______________��
��4���������ڱȽ�Na��Al���������ǿ������ʵ��_________________��
A.����������Ӧˮ����ļ��� B.Na�����1�����Ӷ�Al �����3������
C.������H2O��Ӧ�����׳̶� D.�Ƚ�ͬŨ��NaCl��AlCl3��pHֵ
��5����Ȣ��۵�߳��ܶ࣬�������ǣ�_____________________________���ٺ͢ڶ��������Ӿ��壬���ٱȢڵ��۵�ߣ������ԭ��____________________________��