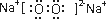��Ŀ����
A��B��C����ѧ��ѧ�г���������Ҹ�������Ԫ����ɣ��ס��ҡ����� ���ֳ����ĵ��ʣ���Щ������͵���֮���������ͼ��ʾת����ϵ����Щת��������Ҫʹ�ô�������
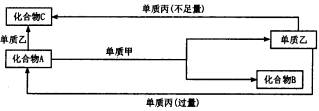
�Իش��������⣺
��1����������ԭ�������dz����������ʣ����dz����ǽ������ʣ���C�Ļ�ѧʽ�� ________��A�ĵ���ʽ��________��A���Ӧ�Ļ�ѧ����ʽΪ________��
��2�������������������dz����ķǽ������ʣ����dz����������ҷ�Ӧ��������Һ��ͨ �������½��У���C�Ļ�ѧʽ��________���ڻ�����A�뵥���ҷ�Ӧ�ĸ��ӷ��� ʽ��________��������C�뵥�ʱ���Ӧ�����ӷ���ʽ��________��������A�뵥�ʼ� �����ʵ���֮��Ϊ2��3ʱ��A���ǡ����ȫ��Ӧ�ҷ�����ͼ��ʾ��ת����ϵ����Ӧ �����ӷ���ʽ��________________��
�𰸣�
������
������
��1��
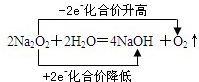
CO �� 2Mg��CO2 2Fe3����Fe =3Fe2�� 2Fe2����4Br����3Cl2=Fe3����2Br2��6Cl��
|

��ϰ��ϵ�д�
 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�
�����Ŀ


 HS-+OH-
HS-+OH- Al��OH��3+3H+����ɫ����Ͱ�ɫ��״��������
Al��OH��3+3H+����ɫ����Ͱ�ɫ��״�������� ��֪A��B��C����ѧ��ѧ�ij������ʣ�������һ��������������ת����ϵ��
��֪A��B��C����ѧ��ѧ�ij������ʣ�������һ��������������ת����ϵ��