��Ŀ����
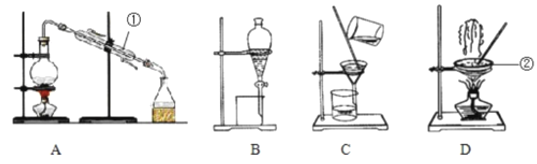
����Ŀ��Ϊ�ⶨij��Ʒ��̼�����Ƶ���������(������������Ȼ���),ijѧ�����������ʵ�鷽��:

(1)��������Ҫ��������_______________��
(2)��Ʒ����_________(������)�����գ���_________(������)����ȴ��
(3)ʵ���в���A������Ϊ_______________��
(4)���պ����Ʒ���ڿ�������ȴ,�����ʵ����_______(����ƫ��������ƫС������������)��
(5)��Ʒ��̼�����Ƶ���������Ϊ_________(����3λ��Ч����)����֪����Ʒ̼�����Ƶ���������Ϊ0.800����ʵ���������Ϊ___________��
(6)�����պ����Ʒ������ϡ�����ܽ�,���ɵ������ڱ�״���µ����Ϊ_____����(��̼��������������0.800����)��
���𰸡�������ƽ ���� ������ ���� ƫ�� 0.768 -4% 640
��������
��1��������ƽ���ܾ�ȷ��С�������λ����ȷ�������������������ǵ�����ƽ���ʴ�Ϊ��������ƽ��
��2������Ӧ���������У����պ����Ʒ���ڿ�������ȴ��̼������ˮ�����Է��ڸ���������ȴ���ʴ�Ϊ����������������
��3�����������������������ٱ仯��ʵ������к��ط����ʴ�Ϊ�����أ�
��4�����պ����Ʒ���ڿ�������ȴ��̼������ˮ�������ʵ����ƫ�ʴ�Ϊ��ƫ��
��5��̼���������ȷֽ�����̼���ƣ����ݻ�ѧ��Ӧ����ʽ2NaHCO3![]() Na2CO3 + H2O + CO2�����㣬��168g NaHCO3�ֽ�ʱ������������62g�����ڼ���3.000 g - 2.150 g = 0.850g������NaHCO3������Ϊ2.303g��̼�����Ƶ���������Ϊ
Na2CO3 + H2O + CO2�����㣬��168g NaHCO3�ֽ�ʱ������������62g�����ڼ���3.000 g - 2.150 g = 0.850g������NaHCO3������Ϊ2.303g��̼�����Ƶ���������Ϊ![]() ��������
��������![]() ���ʴ�Ϊ��0.768��-4%��
���ʴ�Ϊ��0.768��-4%��
��6�����ݻ�ѧ��Ӧ����ʽ2NaHCO3+H2SO4=Na2SO4+2H2O+2CO2������168g NaHCO3�μӷ�Ӧʱ������CO2 44.8L = 44800 mL����3.000g��0.800=2.400g�μӷ�Ӧʱ����CO2 640mL���ʴ�Ϊ640��