��Ŀ����
����Ŀ�������ҹ�ú��������Ա�ʯ�ͺ���Ȼ���ḻ����Դ������úΪ������ԭú��Ϊȼ��ȼ�գ���������Ⱦ������Ч���ֲ��ߣ�������Ҵ����ᳫú���ۺ����á�ʵʩ��ú����������ԭ��֮һ���Ƚ�ú��ˮ�����Ƶ�ˮú��(һ����̼������)���ٴ��ϳɼ״��������Һ̬��Դ��
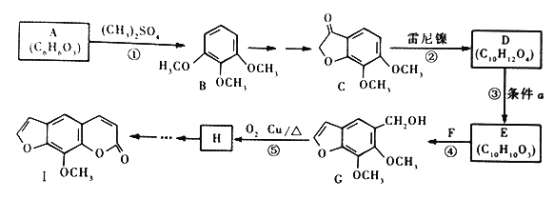
��1����һ����̼�������ϳɼ״��Ļ�ѧ����ʽ��______________________________________��
��2����һ����̼�������ϳ���(CnHm)�Ļ�ѧ����ʽ��__________________________��
��3��������Һ�����ŵ���______________________��
���𰸡� CO��2H2![]() CH3OH nCO��
CH3OH nCO��![]() H2
H2![]() CnHm��nH2O ʹú��������Դ�����ú��ȼ��Ч��
CnHm��nH2O ʹú��������Դ�����ú��ȼ��Ч��
����������1������ԭ���غ���дһ����̼�������ϳɼ״��Ļ�ѧ����ʽ��
��2������ԭ���غ���дһ����̼�������ϳ���(CnHm)�Ļ�ѧ����ʽ��
��3������ԭú��Ϊȼ��ȼ�գ���������Ⱦ������Ч���ֲ��߷�����
��1������ԭ���غ��֪��һ����̼�������ϳɼ״��Ļ�ѧ����ʽ��CO��2H2![]() CH3OH��
CH3OH��
��2������ԭ���غ��֪��һ����̼�������ϳ���(CnHm)�Ļ�ѧ����ʽ��nCO��![]() H2
H2![]() CnHm��nH2O��
CnHm��nH2O��
��3������ԭú��Ϊȼ��ȼ�գ���������Ⱦ������Ч���ֲ��ߣ����������Һ�����ŵ���ʹú��������Դ�����ú��ȼ��Ч�ʡ�

 ��������һ���þ�ϵ�д�
��������һ���þ�ϵ�д� Сѧ��10����Ӧ����ϵ�д�
Сѧ��10����Ӧ����ϵ�д�����Ŀ�����պ�����CO2�ǻ�����ѧ���о����ȵ㣬�Ǽ�������ЧӦΣ������Ҫ;����
(1)��̫���ܵ������£���CO2Ϊԭ����ȡ̿�ڵ�������ͼ��ʾ�����ܷ�Ӧ�Ļ�ѧ����ʽΪ_____________��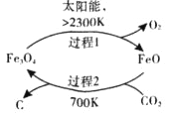
(2)CO2�������⻯�ϳɵ�̼ϩ�����ϳ���ϩ�ķ�ӦΪ
2CO2(g)+6H2(g)==CH2=CH2(g)+4H2O(g) ��H=akJ/mol
��֪:
�� | H-H | C=O | C=C | O-H | C-H |
����/kJ/mol | 436.0 | 745.0 | 615.0 | 462.8 | 413.4 |
��a=________��
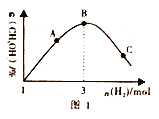
(3)T��ʱ���ں����ܱ������г���1molCO2��nmolH2����һ�������·�����Ӧ:CO2(g)+3H2(g)![]() CH3OH(g)+H2O(g) ��H<0�����������CH3OH ��������������������ʵ����Ĺ�ϵ��ͼ��ʾ��ͼ1��A��B��C �����Ӧ����ϵ��CO��ת����������____(����ĸ)���ж�������____________��
CH3OH(g)+H2O(g) ��H<0�����������CH3OH ��������������������ʵ����Ĺ�ϵ��ͼ��ʾ��ͼ1��A��B��C �����Ӧ����ϵ��CO��ת����������____(����ĸ)���ж�������____________��
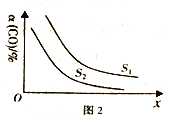
(4) ��ҵ��Ҳ�úϳ���(H2��CO) �ϳɼ״�����ӦΪ2H2(g)+CO(g)![]() CH3OH(g) ��H<0����10L�ĺ������������г���H2��CO�����ʵ����ֱ�Ϊ2mol��1mol�����CO��ƽ��ת�������¶ȡ�ѹǿ�Ĺ�ϵ��ͼ2��ʾ��
CH3OH(g) ��H<0����10L�ĺ������������г���H2��CO�����ʵ����ֱ�Ϊ2mol��1mol�����CO��ƽ��ת�������¶ȡ�ѹǿ�Ĺ�ϵ��ͼ2��ʾ��
��ͼ2��S��������������_________��
����֪300��ʱ������Ӧ�ﵽƽ��ʱ��CO ��ƽ��ת����Ϊ60% �������ƽ����ϵ���ټ���2molCO��2molH2��2molCH3OH �������¶Ⱥ������ݻ����䣬��ƽ���_____(������ƶ����������ƶ������ƶ���)��
(5)��ҵ�ϳ��ø�Ũ�ȵ�K2CO3��Һ����CO2������ҺX�������õ�ⷨʹK2CO3��Һ��������װ��ʾ��ͼ����ͼ��ʾ��

������������CO2��ԭ����________(�����ӷ���ʽ��ʾ)��
������ƽ���ƶ�ԭ��������CO32-��������������ԭ��____________��