��Ŀ����
��.ij��Ȼ��֬A�Ļ�ѧʽΪC57H106O6��1Ħ������֬ˮ��ɵõ�1Ħ�����͡�1Ħ��������֬����B��2Ħ��ֱ������֬����C�����ⶨB����Է�������Ϊ280��ԭ�Ӹ�����ΪC��H��O=9��16��1��
(1)д��B�ķ���ʽ��________________________��
(2)д��C�Ľṹ��ʽ��___________________��C��������___________________��
(3)д����5��̼ԭ�ӵ�Cͬϵ���ͬ���칹��Ľṹ��ʽ��____________________��
��.RCH=CHR�������KMnO4��Һ���Ⱥ��ữ������˫�������������
![]() �����ø÷�Ӧ�IJ��ﷴ�ƺ�̼̼˫��������Ľṹ���ڴ��������£�1Ħ��������֬����B��1Ħ��������Ӧ�����õ�D��E�Ļ���D��E��Ϊͬ���칹�塣��D��E�Ļ���������KMnO4��Һ���Ⱥ��ữ���õ��������ֲ��
�����ø÷�Ӧ�IJ��ﷴ�ƺ�̼̼˫��������Ľṹ���ڴ��������£�1Ħ��������֬����B��1Ħ��������Ӧ�����õ�D��E�Ļ���D��E��Ϊͬ���칹�塣��D��E�Ļ���������KMnO4��Һ���Ⱥ��ữ���õ��������ֲ��
HOOC��(CH2)10��COOH��CH3��(CH2)7��COOH��
HOOC��(CH2)7��COOH��CH3��(CH2)4��COOH��
(4)д��D��E�Ľṹ��ʽ��_________________________________________________��
(5)д��B�Ľṹ��ʽ��_____________________________________________________��
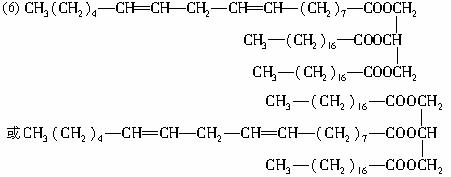
(6)д����Ȼ��֬A��һ�ֿ��ܵĽṹ��ʽ��____________________________________��
(1)C18H32O2
(2)CH3��(CH2)16��COOH Ӳ֬��(��ʮ�����ᡢʮ����)
(3)CH3CH2CH2CH2COOH��CH3CH2CH(CH3)COOH��CH3CH(CH3)CH2COOH��(CH3)3CCOOH
(4)CH3(CH2)7��CH=CH��(CH2)7��COOH
CH3(CH2)4��CH=CH��(CH2)10��COOH
(5)CH3(CH2)4��CH=CH��CH2��CH=CH��(CH2)7��COOH
����:
��.����B�ķ���ʽΪC9nH16nOn����12��9n+1��16n+16��n=280�����n=2������B�ķ���ʽΪC18H32O2��A��ˮ����Ա�ʾ�ɣ�C57H106O6+3H2O��C3H8O3(����)+C18H32O2+2C������ԭ���غ��֪C�ķ���ʽΪ��C18H36O2�����C��ֱ������֬���ᣬ��֪C�Ľṹ��ʽΪ��CH3��(CH2)16��COOH����Ӳ֬�ᡣ����5��Cԭ�ӵ�Ӳ֬���ͬϵ������У�
CH3CH2CH2CH2COOH��CH3CH(CH3)CH2COOH��
CH3CH2CH(CH3)COOH��(CH3)3CCOOH����ͬ���칹�塣
��.�������⣬D��E��Ϊͬ���칹�壬����Cԭ������ͬ�����Ǹ�����KMnO4���ú����ɵ����ֲ����Cԭ����֮����ȣ���D����HOOC��(CH2)10��COOH��CH3��(CH2)4��COOH��E����CH3��(CH2)7��COOH��HOOC��(CH2)7��COOH������˫���������Ȼ�����֮��Ķ�Ӧ��ϵ����֪D�Ľṹ��ʽΪHOOC��(CH2)10��CH=CH��(CH2)4��CH3��E�Ľṹ��ʽΪCH3��(CH2)7��CH=CH��(CH2)7��COOH��B�ķ���ʽΪC18H32O2����֪�������������C=C������һ��C=C��H2�ӳɺ�õ�D����E��ȷ��B�Ľṹ��ʽΪ��
CH3��(CH2)4��CH=CH��CH2��CH=CH��(CH2)7��COOH��

 ѧ�����νӽ̲��Ͼ���ѧ������ϵ�д�
ѧ�����νӽ̲��Ͼ���ѧ������ϵ�д� Сѧ������ҵϵ�д�
Сѧ������ҵϵ�д� ��ʿһ��ȫͨϵ�д�
��ʿһ��ȫͨϵ�д� +3NaOH��C17H35COONa+
+3NaOH��C17H35COONa+
 RCOOH+R��COOH�����ø÷�Ӧ�IJ��ﷴ�ƺ�̼̼˫��������Ľṹ��
RCOOH+R��COOH�����ø÷�Ӧ�IJ��ﷴ�ƺ�̼̼˫��������Ľṹ��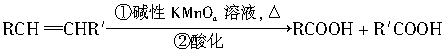 �������ø÷�Ӧ�IJ��ﷴ�ƺ�̼̼˫��������Ľṹ���ڴ��������£�1mol������֬����B��?1molH2��Ӧ�����õ�D��E�Ļ���D��E��Ϊͬ���칹�塣��D��E�Ļ���������KMnO4��Һ�����ữ�õ��������ֲ��
�������ø÷�Ӧ�IJ��ﷴ�ƺ�̼̼˫��������Ľṹ���ڴ��������£�1mol������֬����B��?1molH2��Ӧ�����õ�D��E�Ļ���D��E��Ϊͬ���칹�塣��D��E�Ļ���������KMnO4��Һ�����ữ�õ��������ֲ��