��Ŀ����
��֪ͨ������£�1molC��ʯī����ȫȼ������CO2����ʱ����393.5KJ��1molCO������ȫȼ������CO2����ʱ����283.0KJ������˵����ȷ����
| A��ʯīȼ������CO������Ȼ�ѧ����ʽΪ�� ����2C(ʯī)+O2(g)=2CO(g)��H=-110.5kJ/mol |
| B��C(ʯī)����ȫȼ�գ�����CO2��CO��������ʱ���ɷ���283.0kJ |
| C��C(ʯī)��CO2(g)��Ӧ����CO(g)�ķ�Ӧ�����ȷ�Ӧ |
| D�������ʯ��ȼ������CO2����ų�����������ʯī����ʯī��ɽ��ʯ�ı仯�Ƿ��ȷ�Ӧ |
C
��Ŀ�����������Ȼ�ѧ����ʽΪ��
��C(ʯī)��O2(g)=CO2(g) ��H=��393.5kJ��mol��1
��CO(g)��1/2O2(g)=CO2(g) ��H=��283.0kJ��mol��1
��-�ڵõ��ۣ�C(ʯī)+1/2O2(g)=CO(g) ��H=-110.5kJ/mol
��-�ڡ�2�õ��ܣ�C(ʯī)+CO2(g)=2CO(g) ��H=+172.5kJ��mol��1
A���ɢ�֪������ȷ
B�����ھ�����������������ȷ����������Ҳ����������ȷ
C���ɢ�֪����ȷ
D�����ʯ��ȼ������CO2����ų�����������ʯī��˵�����ʯ�����������ϸߣ���ʯī��ɽ��ʯ�ı仯�����ȷ�Ӧ
��ΪC
��C(ʯī)��O2(g)=CO2(g) ��H=��393.5kJ��mol��1
��CO(g)��1/2O2(g)=CO2(g) ��H=��283.0kJ��mol��1
��-�ڵõ��ۣ�C(ʯī)+1/2O2(g)=CO(g) ��H=-110.5kJ/mol
��-�ڡ�2�õ��ܣ�C(ʯī)+CO2(g)=2CO(g) ��H=+172.5kJ��mol��1
A���ɢ�֪������ȷ
B�����ھ�����������������ȷ����������Ҳ����������ȷ
C���ɢ�֪����ȷ
D�����ʯ��ȼ������CO2����ų�����������ʯī��˵�����ʯ�����������ϸߣ���ʯī��ɽ��ʯ�ı仯�����ȷ�Ӧ
��ΪC

��ϰ��ϵ�д�
 ��������һ���þ�ϵ�д�
��������һ���þ�ϵ�д� Сѧ��10����Ӧ����ϵ�д�
Сѧ��10����Ӧ����ϵ�д�
�����Ŀ


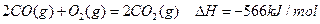
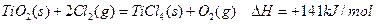
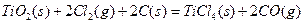 ��
�� =________________��
=________________�� aʱ��lmol H2��ȫȼ������Һ̬ˮ���ų�2
aʱ��lmol H2��ȫȼ������Һ̬ˮ���ų�2 85��8kJ��������1 mol CH4��ȫȼ������Һ̬ˮ��CO2���壬�ų�890��3kJ�������������Ȼ�ѧ����ʽ��д��ȷ���� �� ��
85��8kJ��������1 mol CH4��ȫȼ������Һ̬ˮ��CO2���壬�ų�890��3kJ�������������Ȼ�ѧ����ʽ��д��ȷ���� �� �� ��+2 O2��g��=CO2��g��+2H2O��1������H=-890��3kJ��mol
��+2 O2��g��=CO2��g��+2H2O��1������H=-890��3kJ��mol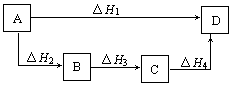
 4
4