��Ŀ����
��֪��N2��g����3H2��g�� 2NH3��g����H����92.4kJ/mol������Ŀǰ�ձ�ʹ�õ��˹��̵��ķ�������ش��������⣺
2NH3��g����H����92.4kJ/mol������Ŀǰ�ձ�ʹ�õ��˹��̵��ķ�������ش��������⣺
(1)450��ʱ����һ��2L���ܱ������г���2. 6mol H2��1mol N2�� ��Ӧ�����ж�NH3��Ũ�Ƚ��м�⣬�õ����������±���ʾ��
| ʱ��/min | 5 | 10 | 15 | 20 | 25 | 30 |
| c(NH3)/mol��L��1 | 0.08 | 0.14 | 0.18 | 0.20 | 0.20 | 0.20 |
�����и�������Ϊ�жϸ÷�Ӧ�ﵽ��ѧƽ��״̬��������__________���������ĸ��
A��������N2��H2��NH3��Ũ��֮��Ϊ1��3��2
B��v��N2����=3v��H2���� C.������ѹǿ���ֲ���
D�����������ܶȱ��ֲ��� E.�����ڻ�������ƽ������������
�����ı�ijһ����������ƽ��ʱn(H2)="1.60mol" ������˵����ȷ����_____________��
A.ƽ��һ�������ƶ� B.�������������м�����һ������H2����
C.�����ǽ������������¶� D.��������С�����������
��2��450��ʱ������һ�ܱ������н��������ϳɰ��ķ�Ӧ�������ʵ���ʼŨ�Ⱥ�ƽ��Ũ�����±���ʾ��
| | N2 | H2 | NH3 |
| ��ʼŨ�ȣ�mol/L�� | 0.2 | 0.3 | 0.2 |
| ƽ��Ũ�ȣ�mol/L�� | a | b | c |
�ٸ����ʵ�ƽ��Ũ�ȿ�����_______________��
A��c="0.5mol/L " B��b="0.5mol/L " C��c="0.4mol/L " D��a=0.3mol/L
��a��ȡֵ��Χ�ǣ�_______________��
��������ѧ����ʽ��ʾ������֮��Ĺ�ϵ��
(I)a��b�Ĺ�ϵ��_______________��
(��)a��b��c�Ĺ�ϵ��_______________��
(3) �������¡��˹��̵������о��������ڳ��¡���ѹ�����������£�N2�ڴ�������������Fe2O3��TiO2��������ˮ�ɷ������з�Ӧ��2N2��g����6H2O(l)
 4NH3��g����3O2��g�����˷�Ӧ�ġ�S__________0���
4NH3��g����3O2��g�����˷�Ӧ�ġ�S__________0���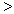 ���� ��
���� �� ����=������ ��H��_____________��
����=������ ��H��_____________������֪��H2��ȼ����Ϊ285.8 kJ/mol��
(1)��0.008mol.L��1.min-1 ; 0.1; �� (ÿ��1��) ��C E (2��) ��CD (2��)
(2)��B(1��) �� 0.1��a��0.3 (2��) ��( I )3a="b+0.3 " (2��)����c2/(a��b3)="0.1" (2��)
(3)�� (1��) +1530 kJ/mol (2��)
����

��ϰ��ϵ�д�
�����Ŀ
 ��һ���¶��£����ݻ�����������м���2mol N2��8mol H2�����������ʹ֮��Ӧ����֪��N2��g��+3H2��g��?2NH3��g������H=-92.2kJ?mol-1��ƽ��ʱ������������ѹǿΪ��ʼʱ��80%��
��һ���¶��£����ݻ�����������м���2mol N2��8mol H2�����������ʹ֮��Ӧ����֪��N2��g��+3H2��g��?2NH3��g������H=-92.2kJ?mol-1��ƽ��ʱ������������ѹǿΪ��ʼʱ��80%��
 9N2��g��+6CO2��g��+6H2O��g����
9N2��g��+6CO2��g��+6H2O��g���� 9N2��g��+12CO2��g��+12H2O��g����
9N2��g��+12CO2��g��+12H2O��g����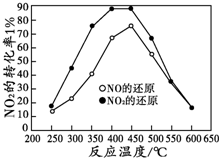
 O2+Hb?CO K=220
O2+Hb?CO K=220 ������һ����Ҫ�Ļ���ԭ�ϣ������Ź�ҵ�����̵ļӿ죬����Ҳ��ɾ�����һ����Ҫ����Ⱦ�����壮
������һ����Ҫ�Ļ���ԭ�ϣ������Ź�ҵ�����̵ļӿ죬����Ҳ��ɾ�����һ����Ҫ����Ⱦ�����壮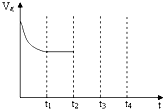 Ŀǰ��ȫ���������Ϊ�����������������������������������Ҫԭ��֮һ���������������ж��ַ������������ϰ�װ��Ч��ת��������ʹ����β���е���Ҫ��Ⱦ�CO��NOx��̼�⻯����������Ӧ�����������ʣ���������β����Ⱦ��
Ŀǰ��ȫ���������Ϊ�����������������������������������Ҫԭ��֮һ���������������ж��ַ������������ϰ�װ��Ч��ת��������ʹ����β���е���Ҫ��Ⱦ�CO��NOx��̼�⻯����������Ӧ�����������ʣ���������β����Ⱦ��