��Ŀ����
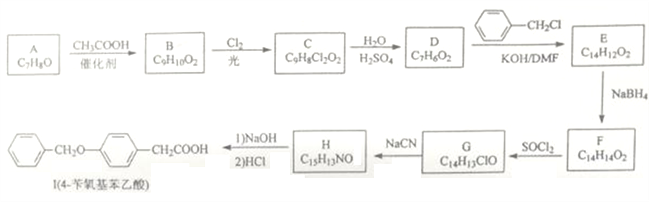
����Ŀ���ɷ��㻯����A�ϳ�ҩ���м���I��һ�ֺϳ�·�����£�
��֪��
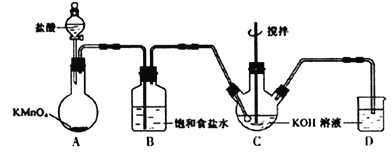
��A��B��C��D�ı�����ֻ������ȡ����������D���ܷ���������Ӧ������FeCl3��Һ������ɫ��Ӧ��
��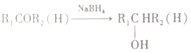
��![]()
�ش��������⣺
(1)A�Ļ�ѧ����Ϊ______________��H�к��������ŵ�������_______________��
(2)G����H�ķ�Ӧ������__________________��
(3)B��C�Ľṹ��ʽ����Ϊ____________��_________________��
(4)C����D�Ļ�ѧ����ʽΪ___________________��
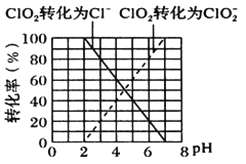
(5)���㻯����X��E��ͬ���칹�壬����ʹBr2/CCl4��Һ��ɫ��������NaHCO3��Ӧ����CO2����˴Ź���������ʾ��������4�ֲ�ͬ��ѧ�������⣬�����֮��Ϊ6��3��2��1��д�����ַ���Ҫ���X�Ľṹ��ʽ��______________��________________��
(6)����������Ϣ��д�����Ҷ���Ϊԭ�ϣ��Ʊ��߷��ӻ�����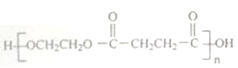 �ĺϳ�·��________________(�����Լ�����)��
�ĺϳ�·��________________(�����Լ�����)��
���𰸡� �Լױ���(4-������) �Ѽ� ȡ����Ӧ ![]()
![]()
![]()
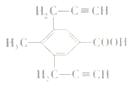
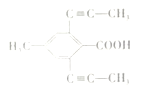
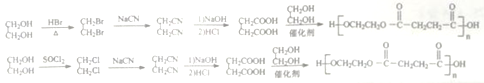
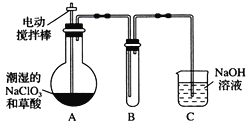
���������� �Ң�A��B��C��D�ı�����ֻ������ȡ����֪AΪ�Լױ���������
�Ң�A��B��C��D�ı�����ֻ������ȡ����֪AΪ�Լױ��������� ��
��![]() ���ݿ�ͼ����Ϣ����֪H ��
���ݿ�ͼ����Ϣ����֪H ��![]() CH2CN
CH2CN
���еĹ�����Ϊ�Ѽ����𰸣��Լױ���(4-������) �Ѽ���
(2)�� 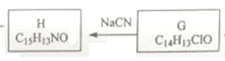 ֪G����H�ķ�Ӧ������ȡ����Ӧ��
֪G����H�ķ�Ӧ������ȡ����Ӧ��
(3)  ��֪AΪ�Լױ��������ᷢ��������Ӧ����B�ṹ��ʽΪ
��֪AΪ�Լױ��������ᷢ��������Ӧ����B�ṹ��ʽΪ![]() ����
����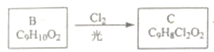 ֪��������ȡ����Ӧ������B�Ľṹ֪C�ĽṹΪ��
֪��������ȡ����Ӧ������B�Ľṹ֪C�ĽṹΪ��![]() ��
��
(4) 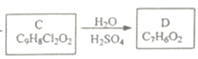 C����D�ķ�����ѧ����ʽΪ
C����D�ķ�����ѧ����ʽΪ![]() ��
��
(5)��D ![]() �Ľṹ��ʽ
�Ľṹ��ʽ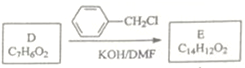 D��E�ķ�Ӧ��ϵ֪��E�ĽṹΪ��
D��E�ķ�Ӧ��ϵ֪��E�ĽṹΪ��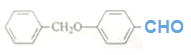 ���㻯����X��E��ͬ���칹�壬����ʹBr2/CCl4��Һ��ɫ��������NaHCO3��Ӧ����CO2����˴Ź���������ʾ��������4�ֲ�ͬ��ѧ�������⣬�����֮��Ϊ6��3��2��1���ַ���Ҫ���X�Ľṹ��ʽ��
���㻯����X��E��ͬ���칹�壬����ʹBr2/CCl4��Һ��ɫ��������NaHCO3��Ӧ����CO2����˴Ź���������ʾ��������4�ֲ�ͬ��ѧ�������⣬�����֮��Ϊ6��3��2��1���ַ���Ҫ���X�Ľṹ��ʽ��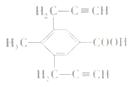
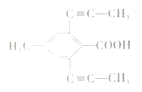 ��
��
(6)����������Ϣ��д�����Ҷ���Ϊԭ�ϣ��Ʊ��߷��ӻ�����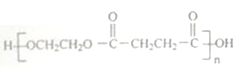 �ĺϳ�·��Ϊ
�ĺϳ�·��Ϊ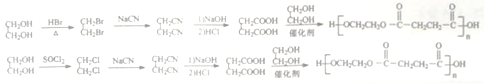 ��
��