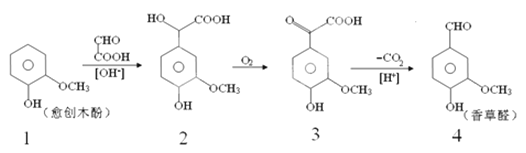
��Ŀ����
����Ŀ��̼���ס����Ԫ���γɵĵ��ʺͻ��������������������Ҫ����;��
(1)���е�ԭ�ӵĵ����Ų�ͼ��ʾ��״̬�У������ɵ͵��ߵ�˳����___(����ĸ)��
A.![]() B.
B.![]()
C.![]() D.
D.![]()
(2)P4S3����������������ӽṹ��ͼ��ʾ��
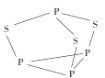
��P4S3��������ԭ�ӵ��ӻ��������Ϊ___��
��1molP4S3�����к��еŵ��ӶԵ���ĿΪ___�ԡ�
(3)��ѧ�Һϳ���һ�������ӡ�N5n+������ṹ�ǶԳƵģ�5��N�ųɡ�V���Σ�ÿ��N���ﵽ8�����ȶ��ṹ���Һ���2�������������˺��ֺϳ���һ�ֺ��С�N5n+���Ļ�ѧʽΪ��N8�������Ӿ���(�þ�����ÿ��Nԭ�Ӷ��ﵽ��8�����ȶ��ṹ)��N8�ĵ���ʽΪ___��(CN)2�м����֮��ļн�Ϊ180�㣬���жԳ��ԣ�������ÿ��ԭ�ӵ�����������8�����ȶ��ṹ����ṹʽΪ___��
(4)ֱ�������������������������������������������ͨ�����ö�����ԭ�����������ģ���ṹ��ͼ��ʾ������n�������������γɵ�������������ӵ�ͨʽΪ___��

(5)̼�����е������Ӳ�ͬ���ȷֽ��¶ȾͲ�ͬ���±�Ϊ����̼���ε��ȷֽ��¶ȺͶ�Ӧ���������ӵİ뾶�����Ž��������Ӱ뾶������̼���ε��ȷֽ��¶������ߣ�ԭ����___��
̼���� | MgCO3 | CaCO3 | SrCO3 | BaCO3 |
�ȷֽ��¶�/�� | 402 | 900 | 1172 | 1360 |
���������Ӱ뾶/pm | 66 | 99 | 112 | 135 |
(6)ʯī�ľ����ṹ��ͼ��ʾ����֪ʯī���ܶ�Ϊ��g��cm-3��C-C���ļ���Ϊrcm��NAΪ�����ӵ�������ֵ����ʯī����IJ���d=___cm��
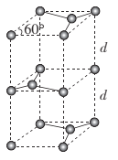
���𰸡�A��C��B��D sp3 10NA ![]() N
N![]() C��C
C��C![]() N PnO3n+1(n+2)- ̼���ηֽ�ʵ�ʹ����Ǿ����������ӽ��̼��������е������ӣ�ʹ̼������ӷֽ�Ϊ������̼�Ĺ��̣����������������ͬʱ�������Ӱ뾶ԽС����������������Խǿ����Ӧ��̼���ξ�Խ���ֽ⣬�ȷֽ��¶�Խ��
N PnO3n+1(n+2)- ̼���ηֽ�ʵ�ʹ����Ǿ����������ӽ��̼��������е������ӣ�ʹ̼������ӷֽ�Ϊ������̼�Ĺ��̣����������������ͬʱ�������Ӱ뾶ԽС����������������Խǿ����Ӧ��̼���ξ�Խ���ֽ⣬�ȷֽ��¶�Խ�� 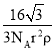
��������
(1)����е���������1s��2s��2p�������ϸߵĹ���е���Խ�࣬��������Խ�ߣ�����2p����ϵ���Խ�ࡢ1s����ϵ���Խ�٣���������Խ�ߣ�����ͼ֪�����ɵ͵��ߵ�˳����A��C��B��D��
(2)�ٸ÷�����ÿ��Sԭ���γ�2�����ۼ��һ�����2���µ��Ӷԣ����ݼ۲���ӶԻ������ۿ�֪Sԭ���ӻ�����Ϊsp3��
�ڸ÷�����ÿ��Sԭ�Ӻ���2���µ��Ӷԡ�ÿ��Pԭ�Ӻ���1���µ��Ӷԣ����Ը÷����йµ��ӶԸ���=3��2+4��1=10����1molP4S3�����к��еŵ��ӶԵ���ĿΪ10NA��
(3)N5n+�ṹ�ǶԳƵģ�5��N�ų�V�Σ�5��N��Ϻﵽ8���ӽṹ���Һ���2��N��N�������������ĽṹΪ��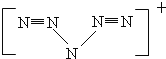 ���ʡ�N5����һ����λ����ɣ����Ի�ѧʽΪ��N8����������ΪN3-��������Ϊ
���ʡ�N5����һ����λ����ɣ����Ի�ѧʽΪ��N8����������ΪN3-��������Ϊ ��N8�ĵ���ʽΪ
��N8�ĵ���ʽΪ![]() ������(CN)2�м����֮��ļн�Ϊ180�㣬���жԳ��ԣ�������ÿ��ԭ�ӵ�����������8�����ȶ��ṹ����ÿ��Cԭ���γ�4�����ۼ���ÿ��Nԭ���γ�3�����ۼ�����ṹ��ʽΪN��C-C��N��
������(CN)2�м����֮��ļн�Ϊ180�㣬���жԳ��ԣ�������ÿ��ԭ�ӵ�����������8�����ȶ��ṹ����ÿ��Cԭ���γ�4�����ۼ���ÿ��Nԭ���γ�3�����ۼ�����ṹ��ʽΪN��C-C��N��
(4)����n��Pԭ�ӵĶ����������ӣ��൱����n�������������ȥ����(n-1)��ԭ�ӣ�Oԭ����Ŀ=4n-(n-1)=3n+1���������Ϊ(-2)��(3n+1)+5n=-(n+2)���ʶ����������ӵ�ͨʽΪ��PnO3n+1(n+2)-��
(5)̼���ηֽ�ʵ�ʹ����Ǿ����������ӽ��̼��������������ӣ�ʹ̼������ӷֽ�Ϊ������̼�Ĺ��̣����������������ͬʱ�������Ӱ뾶ԽС����������������Խǿ����Ӧ��̼���ξ�Խ���ֽ⣬�ȷֽ��¶�Խ�ͣ�
(6)������Cԭ�ӵ���ĿΪ![]() �����Ծ�������Ϊ
�����Ծ�������Ϊ![]() ���辧���ĵױ߳�Ϊacm�������ĸ�Ϊh cm������Ϊd cm����h=2d������ͼΪ
���辧���ĵױ߳�Ϊacm�������ĸ�Ϊh cm������Ϊd cm����h=2d������ͼΪ ����
����![]() ������a=
������a=![]() r����������Ϊ
r����������Ϊ![]() cm2�������ΪV=
cm2�������ΪV=![]() cm3��������
cm3��������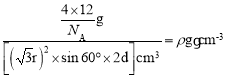 �����d=
�����d=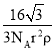 ��
��

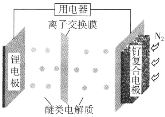
����Ŀ��Zn��һ��Ӧ�ù㷺�Ľ���������п��(��Ҫ�ɷ�ΪZnS��������SiO2������FeS��CdS��PbS���黯�������ʵ�)Ϊԭ���Ʊ�����Zn��ZnSO4��7H2O��������ͼ��ʾ��

����ؽ�������[c(Mn��)��0.1 mol��L��1]�γ��������������pH��Χ���£�
�������� | Fe3+ | Fe2+ | Zn2+ | Cd2+ |
��ʼ������pH | 1.5 | 6.3 | 6.2 | 7.4 |
������ȫ��pH | 2.8 | 8.3 | 8.2 | 9.4 |
��FeAsO4������ˮ��ZnSO4��7H2O������ˮ�������ھƾ���
�ش��������⣺
(1)����1����Ҫ�ɷֳ�SiO2���______�����պ����������Ի�����ɵij���Σ��Ϊ______��
(2)�������ӹ����м���ZnO��������___________��
(3)�Ƶõ�ZnSO4��7H2O��ϴ�ӣ�ϴ�Ӿ���ʱӦѡ�õ��Լ�Ϊ____________��
(4)��Һ�е�Cd2������п�۳�ȥ����ԭ���ӹ����з�Ӧ�����ӷ���ʽΪ___________������ʡȥ����ԭ���������裬ֱ���������������������г�ȥCd2����������________��
(5)�������õ�Cd�������������Ӽ��Զ��ε�أ���ع���ʱ������NiO(OH)ת��ΪNi(OH)2������ʱ��ص�������ӦʽΪ_____________������п��ĵ��Һ�ɷ���______�������ʹ�á�
(6)���Һ����Ԫ����AsO33�����ڣ�����������ʱ��������KMnO4��Һ��KMnO4����AsO33��������Ӧ����FeAsO4��д���÷�Ӧ�����ӷ���ʽΪ___________��